Aetiological Agents in Occupational Asthma
Introduction
Asthma is a chronic inflammatory lung disorder characterised by many symptoms of wheezing, cough and chest tightness and also many cells such as mast cells, T-lymphocytes and eosinophiles considered to have major role in initiation of inflammatory response, airway obstruction and mucous secretion.(1) Functionally the immune system is differentiated into antibodies and other cellular functions. These antibodies are secreted and produced by B-cells which in turn is modulated by T-lymphocytes through its suppressor (CD8+) and helper (CD4+) functional cells. Activation of T- cells results in a generation of immune responses through recognition of antigens, however T- cells recognise only soluble antigens which is processed by accessory cells in histocompatibility complex HMC.(2)
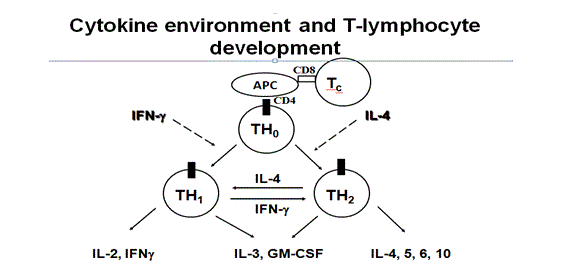
Fig: 1 cytokines and T cells activation
These accessory cells considered important in immune response and secretions of interleukin’s -1(IL-1) which is T- cells stimulatory cytokines. Antigens, which are bound to HMCII, are recognised by CD4+ cells, whereas HMCI bound antigens are CD+8 cells.(3) Antigen presenting cells, such as dendrite cells, cause airway sensitisation as these cells capture allergens process them into lymph node and present selected epitopes of these allergens to HMCII to CD+4 cells of T-cells receptors. High affinity receptors of dendritics cells have a major role in an allergen’s uptake in lungs. When presenting antigens, cytokines decides whether Th0 differentiation is biased towards Th1 or Th2 cells with secretions of IL-2. The co-stimulatory molecule B7-2 located on APC (antigen presenting cells) may interact with CD28 and results in presentation of CD+4 towards the Th2. However, two subsets of CD+4 cells have been observed in mice lungs and both secrets IL-3 and GM-CSF. Th1 secretes IL-2 which stimulates T- lymphocytes, proliferation of macrophages and IL-Y which results in inhibition of IgE synthesis and B-cells activation and causes delayed hypersensitivity. (4) However, Th2 secrets IL-4, IL-5, IL-9-L, IL-10 and IL-13 but not IL-2 and IFN-y and causes IgE mediated type of hypersensitivity reactions. However these Th1-2 cells are not present in the human lung. (1) After T-cells activation by antigens it secretes large numbers of cytokines, which can differentiate and grow into other leukocytes. In this case activated CD4+ cells act as both inflammatory and helper cells at the same time. Allergen sensitisation is caused by repeated exposure to the same allergens which are responsible for the amplification of Th2 response and release of cytokines, especially IL-3, lL-4 IL-5, 9-IL, IL-13 and GM-CSF, which are responsible for chronic airway inflammation. (5) Activation of these allergens through cross-linking of IgE present on mast cells results in a release of inflammatory mediators, which leads to initiation of inflammatory cascade through different pathways. T- Cells can also directly contribute in the activation of immune response or through other events which are responsible for asthma exacerbations. (18)
1. Occupational asthma
Basic principles of occupational asthma (OA) are the same as for non-workers asthma, although it is caused by inhalation of allergens at workplace and counts for 25% or more than of non OA. There are two types of OA; immunological asthma, which involves the latency period, and acquire immunological sensitisation due to repeated exposure to allergens, whereas non immunologic occurs without a latency period after acute exposure to high concentration of irritants. (6) There are many factors which contribute in inducing OA such as genetics, behavioural and environmental factors. There are more than 200 causative agents of OA; some are classified into high molecular weight (HMW) compounds and low molecular (LMW) compounds. (7)
Routes of exposure are generally the respiratory tract, also considered the primary site of immune response. However by reducing the exposure through this route it still occurs at the same frequency. (8) It means allergens can induce asthma other than respiratory route. For example skin exposure to isocynate can cause Th2 sensitisation which can generate inflammatory responses upon inhalation of certain allergens. It was also observed that in some cases at low concentrations these allergens may have a greater response than at high concentrations, which show that length and intensity of exposure also have a role in OA. (9)
1.1. Immunological asthma
Sensitiser induced asthma is believed to act through an IgE dependent mechanism due to exposure to HMW and LMW compounds. HMW (proteins and flour) act as functional antigens. Cross-linking of these allergens with specific IgE antibody causes activation of a cascade of events which results in inflammatory response. For example, baker’s asthma is caused by inhalation of wheat, which in fact does not occur due to wheat sensitisation but which may be caused due to recognition of other allergens present in wheat. (1) For example it is hypothesised that endogenous thioredoxin present in human lungs may have a cross-reactivity with wheat thioredoxin and B- cell epitopes sharing by cereal and human thioredoxin may be the reason of cross-reactivity. This may cause activation of other mediators and inflammatory responses without external exposure and results in the development of baker’s asthma and deterioration of lung inflammation. (10)
LMW agents such as trimellitic anhydride (TMA), nickel and other metal salts also play a role in inducing OA. These LMW compounds first have to react with specific antibodies to produce functional allergens. (19) After sensitisation, it has been observed in skin prick tests that antigen recognition is not only due to TMA hepaten epitopes but new antigens formed during conjugation of TMA to proteins. For example, cross-reactivity of inhaled TMA with IgE may generate trimellityl modified IgE conjugates which acts as antigenic target for antitrimellityl IgE antibodies and results in cross-linking of mast cells activation. (11) This may be due to the specificity of anhydrides structure or ring formation, which is considered a critical determined of IgE mediated sensitisation.(6)
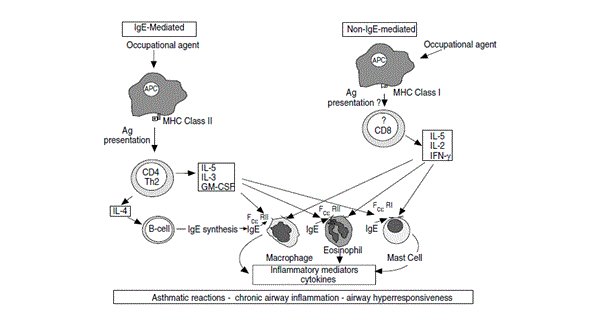
Fig: 2 Mechanism of IgE-mediated and non- IgE mediated asthma
Activated T lymphocytes, CD+8, high concentrations of IL-5 and mast cells have been observed in bronchial mucosa in cell mediated asthma. It was observed that most of the T- cells in TDI induced asthma were CD+8, which produced IL-5 in significant amounts and also in cedar induced asthma T-lymphocytes from peripheral blood of patients have shown secretion of IL-5 and INF after conjugation with plicatic acid and human serum albumin. (12) Cytokines production in this way shows compatibility with airway mucosal oesinophiles. In other examples, tissue culture from peripheral blood of TDI asthmatic patients have shown no secretion of IL-5 or 4. However these cells synthesised TNF alpha which is IgE independent pro-inflammatory cytokine and C-C chemokine mononuclear chemoattractant proteins-1 (MCP-1). The above mentioned studies did not show the possibility that bronchial IgE could be involved when systemic IgE is not present. (13)
1.2. Non immunological
Non immunological mechanism is not completely known yet, but it may occur due to exposure to high concentrations of irritants present at or outside the workplace which can mediate the onset of new asthma and results in airway obstruction and inflammatory responses. (4) For example RADS (Reactive Airway Dysfunction Syndrome) caused by exposure to irritants. Histopathological changes observed in RADS are similar to typical asthma and hypothetically it is observed epithelial damage by irritants such as fumes or smoke causes activation of noncholinergic and nonradrenergic pathways through axon reflexes and activation of neurogenic inflammation. (18)
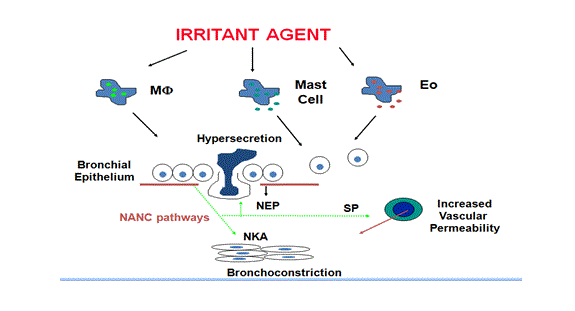
Fig: 3 Mechanism of non immunological asthma
Inflammatory responses may persist for a long period after the epithelial damage due to the secretion of pro-inflammatory mediators. (1) Damaged epithelium also makes easier for irritants to cross the epithelium and leads to sensitisation and development of asthma. Patients observed for RADS have shown symptoms like bronchial hypersensitivity with specific symptoms of dyspnoea cough and wheeze, although none of those previously had respiratory disease. The presence of specific IgE can be very useful for the diagnosis of OA. (14)
1.3. Genetic mechanism
Researches have been trying to establish a link between asthma phenotype and allelic polymorphic genetic markers. It was observed that HLA II has a specific role in asthma. These molecules encoded by HMC gene can affect the immune response to an antigen. Due to different classes of HLA molecules, it can alter the ability of a molecule to bind to peptides and affect the T-cells recognition. (5) For example, asthma induced by LMW and IgE mediated show differences in their association with HLA II allele. Positive association was observed in IgE mediated asthma with HLA II. However, low frequency of DR4 haplotype was observed in isocyanate induced asthma. This low responsiveness can be linked to DQ allele and can be mediated by CD+4 cells which in turn causes CD+8 activations. However haplotypes causes the upregulation of immune response and affect the ability of allergens to enter into the lungs. (15)
Differences in positive and negative association of HLA II was observed in hypervariable amino acid residue 57 in DBQ1 allele and found that residue 57 has a role in disease susceptibility. (16) HLA II also important in antigen presentation therefore, correct binding between amino acids and antigens is necessary for functional antigen presentation. However residue 57 is negatively charged, it can either interact directly with TDI itself or it can modify the HMC II structural activity or can act as peptides which are presents on the APC surface cells in such a way that T-cells recognise these modified peptides as foreign and leads to sensitisation. ( 20) Therefore on the basis of these observations recognition variations in amino acid residue can be used as a powerful tool for secondary prevention of TDI induced asthma. (17)
Conclusion
Mechanism of OA provides a significant step in the controlling and management of disease. Causal agents of OA provide a great structural activity relationship to understand the airway sensitization. A dose response relationship was also observed in many allergens with the level and intensity of the exposure. (1) It was also observed that respiratory sensitisation in OA can be induced by routes other than respiratory. Genetics, along with environmental factors, may change the susceptibility to develop asthma, changing the impact of the gene on complex phenotypes. It means combination of genes and environment can have profound effect in developing OA than their individual. (4)
However, the mechanism of OA induced by LMW still remained unclear. Several studies have shown the role of T-cells and cytokines in OA by LMW agents is not similar to those in atopic asthma. Therefore, further studies are required to describe the relationship between immunologic and inflammatory responses in inducing OA. (5)
References
1. Mechanisms of occupational asthma Piero Maestrelli, MD, Piera Boschetto, MD, Leonardo. Fabbri, MD, and Cristina. Mapp, MD. (J Allergy Clin Immunol 2009; 123:531-42.)
2. Overview of diisocyanate occupational asthma. Jonathan A. Bernstein. Toxicology 111 (1996) 181-189.
3. Occupational asthma Stuart M. Brooksa’.Toxicology Letters 82-83 (1995) 39-45
4. Occupational Asthma: A Review. Lynda J. Lombardo and John R. Balmes. Environ Health Perspective 1 08(suppl 4):697-704 (2000
5. Human Leukocyte Antigen Associations in Occupational Asthma Induced by Isocyanates. C. E. MAPP, A. BALBONI, R. BARICORDI, and L. M. FABBRI. Vol 156. pp S139–S143, 1997
6. Occupational asthma. Emil J. Bardana, Jr, MD, CM Portland, Ore. J Allergy Clinical and Immunological 2008; 121:S408-1
7. Occupational asthma. Katherine M Venables, Moira Chan-Yeung. Lancet 1997; 349: 1465–69
8. Occupational Asthma Caused by Chromium and Nickel. María Jesus Cruz, Roser Costa, Eduard Marquilles, Ferran Morell, and Xavier Muñozc. Arch Bronconeumol. 2006;42(6):302-6
9. Occupational asthma: Current concepts in pathogenesis, diagnosis, and management Mark S. Dykewicz, MD. (J Allergy Clin Immunol 2009;123:519-28.)
10. Occupational asthma by Anisakis Simplex. A. Armentia, MD, Phd, at al. (J Allergy Clin Immunol 1998;102:831-4.
11. Mechanisms of Occupattional Asthma induced byisocyanates. FREDERIC OESCHAMPS, ALAIN PREVOST. FRANCOIS SERGE KOCHMAN . 48-78(97)00047
12. Mechanisms and pathology of occupational asthma C.E. Mapp, M. Saetta, P. Maestrelli, A. Di Stefano, P. Chitano, P. Boschetto, A. Ciaccia, L.M. Fabbri.Eur Respir J, 1994, 7, 544–554
13. Occupational Asthma. Cristina E. Mapp, Piera Boschetto, Piero Maestrelli and Leonardo M. Fabbri. Vol 172. pp 280–305, 2005.
14. Pathogenesis of occupational asthma. J. Sastre, O. Vandenplas, H-S. Park. Eur Respir J 2003; 22: 364–373
15. Mechanism of Occupationla asthma. Not all allergens are equal.Jean F. Regal, at al.2007,7,12:165-171
16. Mechanisms of occupational asthma. Cristina Mapp; Piera Boschetto; Deborah Miotto; Edoardo De Rosa; and Leonardo M Fabbri. Ann Allergy Asthma Immunol 1999;83:645– 664.
17. The role of genetic factors in occupational asthma. C.E. Mapp. Eur Respir J 2003; 22: 173–178
18. Mechanisms of occupational asthma. p. MAESTRELLI, M. SAETTA, C. MAPP and L.M. FABBRI. Clinical and Experimental Allergy, 1997, Volume 27, Supplement 1, pages 47-54.
19. A new mechanism for immunologic initiation of asthma. Allen P. Kaplan. February 1, 2005 vol. 102 ,no. 5, 1267–1268.
20. Overview of diisocyanate occupational asthma. Jonathan A. Bernstein. Toxicology 111 (1996) 181-189