Batch distillation as a highly advantageous tool for the separation of volatile liquids
Introduction
Distillation is commonly used in modern industry to separate miscible volatile liquids (Schaschke, 2014). This separation method takes advantage of the fact that different boiling temperatures are associated with different chemicals. Batch distillation is a form of distillation during which two liquids with different boiling temperatures are vaporised and subsequently cooled down to produce a new mixture with a quantity of one of the liquids higher than in the initial mixture (Schaschke, 2014). The process does not presume the introduction of additional liquids in the heating vessel, and, for this reason, more volatile components will be gradually removed from the vessel as the mixture is boiled. When comparing batch distillation with other purification techniques, it should be indicated that the method is highly advantageous in cases when purification of small quantities of chemicals is necessary (Schaschke, 2014). The batch distillation process is schematically represented in the figure below:
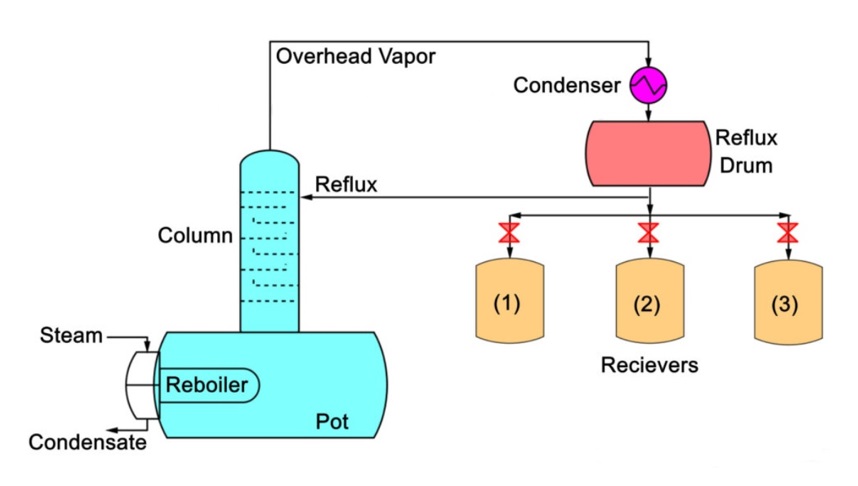
Figure 1: Batch distillation process, adapted from (Backhurst, 2002)
According to the schematics in figure 1, equipment for the batch distillation process included a reboiler (a stirred batch reactor or a still pot), a packed column, an overhead condenser, a reflux drum, and a series of receivers (Backhurst, 2002). During its normal mode of operation, energy is applied to the pot in the form of steam that passes through the coil, producing a condensate. As energy is delivered to the pot the separating liquid starts to boil. The respective overhead vapour is cooled down in the condenser with the generated liquid removed at the predefined time intervals using specialised receivers (Backhurst, 2002).
The versatility of the batch distillation allowed its implementation in various industries to carry out effective water removal/drying, changes of solvent between different stages of chemical synthesis, solution concentration before crystallisation, temperature control during chemical reactions, solvent recovery, and fractional separation of complex mixtures (Shallcross, 2017). Taking into consideration the indicated application areas it is clear that batch distillation is of paramount importance to the industrial purification of hydrocarbons, fine synthesis of new catalysis and development of novel pharmaceutical products (Shallcross, 2017). The aim of the work is to discuss current applications of batch distillation in the modern industry.
Discussion
The number of publications on batch distillation in ScienceDirect has considerably increased during the last decade (ScienceDirect, 2021). According to the database the process was optimised to meet specific industrial needs, aid optimisation of the production, support purification processes or increase yields of a chemical reaction through timely removal of intermediaries. Specifically, the number of publications on the topic increased from approximately 800 in 2010 to more than 2000 in 2021 pointing out to an increasing interest in this form of purification from the global scientific community (ScienceDirect, 2021).
Considering specific examples of using batch distillation, applications of the technique in the petrochemical industry should be put forward (Long, et al., 2020). The respective hydrocarbon mixtures contain chemicals with similar volatility or are characterised by a narrow composition range. For these reasons, an additional rectification compartment was necessary between the still and the condenser (Long, et al., 2020). In addition to the indicated rectification compartment, it is required to introduce specialised overhead facilities that can control the separation of individual components and sustain the necessary reflux ratio during the processing of heterogeneous azeotropes (May-Vazquez, et al., 2020). Consequently, depending on the chemical nature of the separated compounds and processing conditions various methods can be used to carry out batch distillation. In particular, the most widely used methods are presented by utilisation of repetitive total reflux, reducing processing time through changes in the reflux ratio and changing the reflux ratio while maintaining constant product flow (May-Vazquez, et al., 2020).
Batch distillation of hydrocarbons is heavily used in the modern petrochemical industry. For instance, in Chevron’s DPST laboratory in Richmond, automatic batch distillation was developed to enhance the processing of crude oil (Ingham & Sverdlov, 2019). The goal of the respective project was to establish a set of specific control parameters that would ensure an effective liquid boil-up rate by automatically increase the pot temperature to the necessary levels, while at the same time reducing pressure in the system. It was aimed to sustain the operating limits of the column’s condenser to ensure stable outflow of the targeted distillation product. To address the indicated goal of simultaneous variable, control the company made two improvements in the batch distillation setup (Ingham & Sverdlov, 2019). The first improvement was the utilisation of the Proportional-Integral-Derivative (PID) algorithm to support single variable control loops. The second improvement was the integration of oil cumyld, % vs temperature curves for the determination of setpoints of the analysed PID loops. As a result, it became possible to adjust the PID control targets during the distillation process, keeping the distillation rate within the processing capacity of the apparatus. In particular, the described operation conditions ensured that hydrocarbon vapours were able to change into a liquid with no flooding of the distillation column (Ingham & Sverdlov, 2019).
In addition to the application of batch distillation in oil refining, the method can also be used to increase the yields of many industrially important products. For instance, Aqar and co-workers used batch distillation to optimise the generation of methyl palmitate (Aqar, et al., 2021). Industrial quantities of methyl palmitate are necessary for the production of animal feeds, plasticisers, lubricants, resins, stabilisers, wetting agents, emulsifiers and detergents. The indicated range of applications became possible due to the anti-inflammatory as well as anti-fibrotic properties of methyl palmitate (Thermo Fisher Scientific, 2021). Aqar and co-workers highlighted that formation of methyl palmitate was not investigated in the past using batch distillation processes due to considerable challenges associated with keeping both methanol and palmitic acid mixed in the reaction vessel. In particular, methanol is a high volatile organic liquid that quickly travels up the distillation column upon heating (Aqar, et al., 2021). The indicated problem was solved by using a semi-batch reactive distillation column, where additional methanol was provided to the reaction vessel as the temperature increased. In this case, because methanol was adequately supplied to the system, water was the main contaminant that travelled up the column and was removed by using distillation tanks. The performance of the described column was assessed in terms of energy consumption and conversion. As a result, 52-80% improvement in the formation of methyl palmitate was observed (Aqar, et al., 2021).
Conclusions and future work
Publications on batch distillation are not limited to the outlined above, however, discussion of all possible variations of the method and their applications is beyond the goal of the work. In conclusion, it was shown that batch distillation may be used to increase the performance of many important processes that are utilised by the modern chemical industry. For instance, batch distillation can be automated to ensure the maximum efficiency of hydrocarbon processes with minimal energy input. The process can also be altered to reduce the evaporation of specific highly volatile chemicals, increasing the yield of distillation-based chemical reactions. As a result, batch distillation may be considered for both purification and synthesis of new chemicals. Looking at possible expansion of the technology application computer-based simulation of batch distillation using Aspen HYSYS can be put forward (AspenTech, 2021). The program can calculate the dimensions and operation conditions of distillation columns (AspenTech, 2021)
Reference list
Aqar, D., Abbas, A., Patel, R. & Mujtaba, I., 2021. Optimisation of semi-batch reactive distillation column for the synthesis of methyl palmitate. Separation and Purification Technology, 270(September), pp. 1-15.
AspenTech, 2021. Aspen HYSYS. [Online]
Available at: https://www.aspentech.com/en/products/engineering/aspen-hysys
[Accessed 27 August 2021].
Backhurst, J., 2002. Chemical Engineering: Solutions to the Problems. London: Butterworth-Heinemann.
Ingham, H. & Sverdlov, G., 2019. The benefits of automatic batch distillation. [Online]
Available at: https://www.hydrocarbonprocessing.com/magazine/2019/june-2019/process-control-and-instrumentation/the-benefits-of-automatic-batch-distillation
[Accessed 27 August 2020].
Long, N. et al., 2020. HETP measurement using industrial-scale batch distillation. Chemical Engineering and Processing – Process Intensification, 148(February), pp. 1-16.
May-Vazquez, M. et al., 2020. Development of a mass transfer model for the rate-based simulation of a batch distillation column. Computers & Chemical Engineering, 140(September), pp. 1-14.
Schaschke, C., 2014. A Dictionary of Chemical Engineering (Oxford Quick Reference). 1st ed. London: OUP Oxford.
ScienceDirect, 2021. ScienceDIrect: Search for peer-reviewed journal articles and book chapters. [Online]
Available at: https://www.sciencedirect.com/
[Accessed 27 August 2021].
Shallcross, D., 2017. Chemical Engineering Explained: Basic Concepts for Novices. 5th ed. London: Royal Society of Chemistry.
Thermo Fisher Scientific, 2021. Methyl Palmitate. [Online]
Available at: https://www.alfa.com/en/catalog/L05509/
[Accessed 27 August 2021].