Essay on Action Potentials
Number of words: 706
The paper presents all phases for the action potential. Within it, it contains the various ions that move and their respective direction. At the same time, it articulates the mechanism involved in the generation of action potential and how the brain interprets this activity. Besides, the paper explains how analgesics influence the action potential. The document utilizes a scenario where lidocaine given by a dentist impacts the action potentials and how it might numb tissues and affect the cardiac muscle.
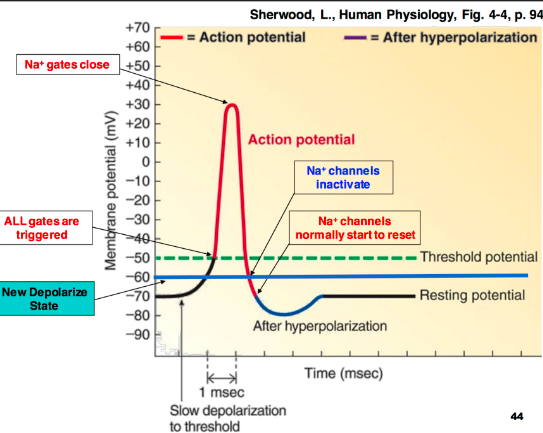
Retrived from: (Rodriguez-Falces et al., 2020).
An action potential is typically the reversal of the membrane potential therein; the membrane potential alter to +30mV from -70mV. The action potential majorly has three phases; depolarization, hyperpolarization, and repolarization (Mojoo, 2015). Whenever the membrane in the hillock axon of the neuron achieves its threshold, a rapid change in the potential membrane happens in a representation of action potential. The depolarization phase (rising phase) results whenever the positively charged ions of sodium rapidly rush via the voltage-gated channels into the neuron. While the additional sodium gets in, the potential membrane ideally reverses polarity. Thus, the change in the polarity formulates a positive value of about +40mV.
Ideally, the repolarization phase (falling phase) results from the reduced closure of the channels of sodium ions and the entrance of the voltage-gated potassium voltage-gated mediums. Consequently, the membrane permeability to sodium elapses to the resting stages. Whenever the entry of sodium get declined, the reduced voltage-gated potassium channels open, and the ions of potassium rush from the direction of the cell (Mojoo, 2015). That expulsion restores the negative localized potential membrane of the cell. Lastly, the hyperpolarization phase occurs when some potassium channels remain open while sodium channels get reset. An instance of rising potassium permeability leads to efflux of excess potassium before the closure of potassium channels, and that appears like a slight dip spike.
The neuron that emits nerve impulse or the action potential gets generated through the mechanism of voltage-gated channels, which get embedded within the plasma membrane of the cells (Mojoo, 2015). Accordingly, the media reach shut level whenever the potential membrane reads negative, the cell’s resting potential. However, it rapidly sets whenever the membrane potential gets increased to a particular voltage frequency. For instance, whenever the medium opens, it permits inward flow for sodium ions, thus altering the gradient of electrochemical ions. The instant influx of sodium reverses the plasma membrane polarity, making potassium channels activated (Rodriguez-Falces et al., 2020). That will encourage an external current for potassium ions, returning the electrochemical gradient to the resting state. The brain conveys these messages through the communication of neurons. Remarkably, these are neurotransmitters that get released within the synapse and align themselves on the receptors of the potential membrane. Thus, the action potential in the subsequent release of neurotransmitters enables the communication between neurons.
The application of analgesics to the peripheral nerve leads to the blockage of the action potential propagation by inhibiting the voltage-gated channels of sodium ions. Such inhibition leads to binding of the inner pore site of the medium accessible from the opening of the cytoplasm.
Lidocaine leads to the decreased generation of the action potential. Thus, making it generate lower or no action potential (Rodriguez-Falces et al., 2020). Lidocaine causes numbness on tissues by blocking the signals of pain towards the brain. That will lead to temporary numbness on the place local anesthetic gets placed. Lidocaine decreases the rate of depolarization for the cardiac tissue. And as a result, it is used to treat ventricular arrhythmias. The cardiac action potential amplitude decreases during a higher concentration, and the conduction velocity gets reduced.
References
Majoo, F. (2015). Anatomy & Physiology Chapter 11 Part A: Fundamentals of the Nervous System and Nervous Tissue Lecture. Retrieved from: https://www.youtube.com/watch?v=W0-80WT5G_s.
Rodriguez-Falces, J., Malanda, A., Lavilla-Oiz, A., & Navallas, J. (2020). Recovery of the first and second phases of the M wave after prolonged maximal voluntary contractions. Journal of Electromyography and Kinesiology, 50, 102385.