Essay on Determination of the Glucose Content of Orange
Number of words: 2608
Aims
In this experience, we shall determine the content of the glucose in citrus fruits such as orange. The experiment will cover all the different classifications of sugars and the role they perform in the body. The investigation will also discuss additional suggestions and alternative methods that can be used to determine the content of glucose of an orange.
Introductions
Carbohydrates
Sugars and starches from carbohydrates. Also, carbohydrates can be defined as compounds of carbon, oxygen, and hydrogen. Carbohydrates can be classified as simple forms such as sugars and complex forms Kwon as starches and fiber. During the body metabolic process, the sugar and starches are broken down to form glucose. Glucose is a simple sugar that can be utilized by the body cells.
In most cases, simple carbohydrates are found in the natural sugars. Simple carbohydrates are more straightforward to be handled by the body cells since they are less complicated (Banauch et al., 2017). The primary sources of these simple carbohydrates are fruits and natural sugars.
On the other hand, complex carbohydrates are frequently monosaccharides units that are bonded together by covalent bonds. The disaccharides consist of two to ten simple units of natural sugar. On the other hand, polysaccharides comprise hundreds and thousands of monosaccharides, which are closely associated. Complex carbohydrates have more metabolic energy compared to simple carbohydrates. Metabolically, the carbohydrates are broken down through the respiration process to yield power. The main functions of carbohydrates are energy production. The carbohydrates supply energy to all body cells (Banach et al.;, 2005). The glucose and fatty acids present in the carbohydrates are the primary sources of power in the body.
Types of sugars
Naturally, there many types of sugar used in the body to generate energy. Sugar is the primary source of energy in all animals’ bodies. Biologically, there are several types of sugars, each performing a specific function in the body. These types include reducing sugars, none reducing sugars, glucose, sucrose, and many others. Sugars can be grouped as reducing or non-reducing sugars. In this case, reducing sugar is pure and is free from aldehyde, which acts as a reducing agent. on the other hand, none reducing sugars are impure; hence they contain aldehyde; therefore, it cannot act as a reducing agent. Different types of sugars have different sugar contents. Citrus fruits such as oranges contain 100g of glucose. Glucose plays a crucial role in the body; after being broken down by enzymes, it provides the energy that enhances cells” growth and divisions.
Importance of glucose in the body
Glucose is the primary source of energy in the human body. Every cell requires energy for development and division. For example, red blood cells need the power to distribute oxygen gas in all parts of the body. The cells cannot function unless there is some energy. The glucose must be deaminated in the liver to form simple compounds for the body cells’ absorption. Glucose plays a crucial role in human brain functions. The human brain contains many neurons that always need glucose as it performs its functions. Thinking, learning, and remembering requires a lot of energy.
During working, skeletal muscles require a large amount of glucose in glycogen for energy production. The complex glucose must be broken in glycogen form to be utilized by the forces during physical exertion. For good health, people should take in foods rich in carbohydrates to energy maintain the strangeness and fitness of the body. They are eating more fruits such as oranges will Défense the body cells against diseases associated with a lack of carbohydrates. Also, taking foods rich in glucose will strengthen the ability to think and recall as the brain involves a high amount of energy to performs its functions.
Qualitative and quantitative determination of glucose
In the qualitative determination of carbohydrates, the glucose is based on the reducing properties. The reducing power is due to the presence of reducing aldehyde. In glucose analysis, the benedict’s solutions are used as a reagent. The unspecified Molisch’s test for glucose is one example of qualitative research (Dubowskie et al.; 2015). In qualitative determination, the quality of glucose is considered as the final result. The qualitative method enables the researchers to get the correct rate of certain carbohydrates is given food. In qualitative analysis, the glucose reduces copper sulphate in Benedict’s reagent under necessary conditions, and red precipitates are formed.
Quantitative determination of data is frequently used in comparison with qualitative method technique. In this experiment, a quantitative determination is used to identify the type of sugar present in the glucose. To determine this, a measured amount of benedict’s solution is added to glucose. The red precipitate is observed, thus indicating the presence of glucose. The quantitative analysis method is mostly used since it gives the correct results.
Principle of glucose oxidase
Glucose oxidase involves catalyzes, which speeds up the reactions. The process of breaking long chains of glucose into small chains is called the oxidation process. The long chains of glucose are broken into gluconate, which can be utilized by the body cells; the glucose must react with oxygen molecules to give out carbon dioxide and some water molecules. The oxidation reactions equations are.
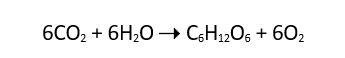
UV-Vis spectroscopy for quantitative analysis
In this research, a kit of quantitative, enzymatic analysis of glucose in carbohydrates and other materials is used in combinations with UV. A spectrophotometer is the most used glucose measurement device because of its speed and ease to use. The spectrophotometry technology can be used in this experiment to measure the content of glucose in the carbohydrates. The necessary code of the method is that it uses the light of a specific wavelength.
Reagents/materials
Spectrophotometer
GOD- PAP reagent
Orange pieces
1mM glucose
Distilled water
Pipette
Centrifuge tubes
Volumetric flask
Cuvettes
Risk assessment of reagents
During the determination of the glucose in the lab, safety precautions should be observed. The reagents should not be handled with bare hands since some are corrosive. After using the reagents, they should be tightly closed because some are explosive. The reagents should be returned in their respective racks to avoid any confusion. No one should be eating when handling the reagents.
PPEs for health and safety
Gloves
Eye and foot protective
Hearing protective devices
Masks
Preparation of the orange extract
Weigh out accurately about 2.5g of this segment and record the extract weight
Place the orange piece in the blender with 40ml distilled water and homogenize for few minutes
Transfer all of the homogenates to 50ml plastic centrifuge tubes, and make sure the volume is equal in each box to your initial.
Carefully decant the supernatant through a filter in a funnel and into the 100ml volumetric flask provided.
Makeup to the mark with distilled water and mix thoroughly. This is the stock orange extract that should be used in this experiment.
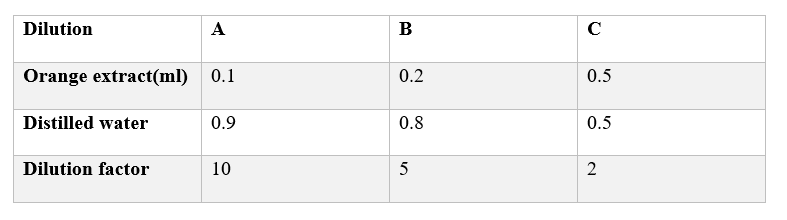
Preparation of the orange dilutions
Label 3 small micro-centrifuge tube that is A, B, and C.
Make three dilutions of orange extract using the corresponding amount of stock orange prepared in the above
Mix each dilution by vertexing
GOS-PAP methods
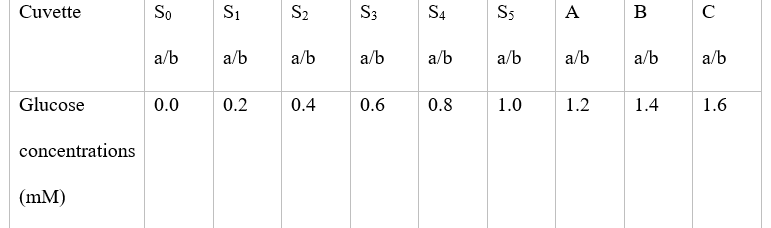
Set up the series of cuvettes as shown in the table below.
The samples marked S0-S5 are the calibration standards, and these should be set up in duplicate.
Use the 1mM glucose standard provided to set up the series calibrations series.
The unknown (A, B, and C ) should be assayed in duplicate.
Use diluted orange extracts for these samples. Cuvettes should total 18.
Results
Standard glucose concentration
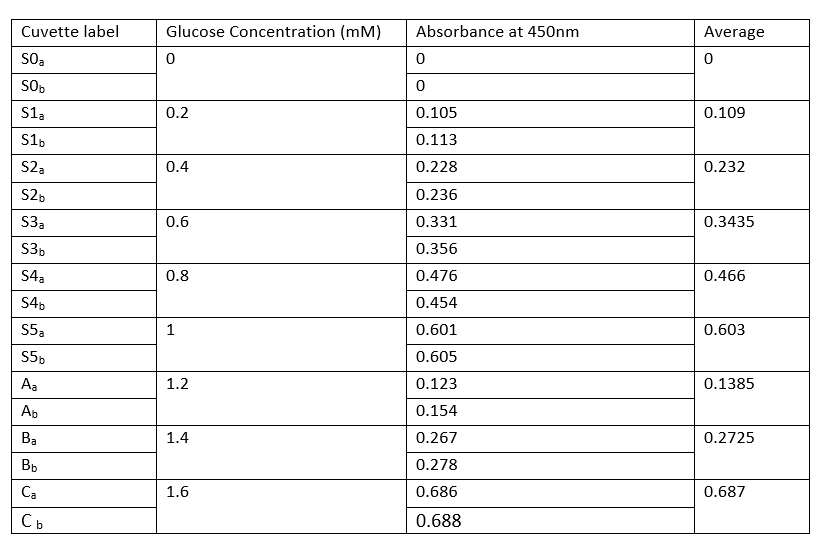
The concentration of glucose increases drastically from one level to the other. As the concentration increases, the reaction rate still increases due to increased enzymes acting on the glucose.
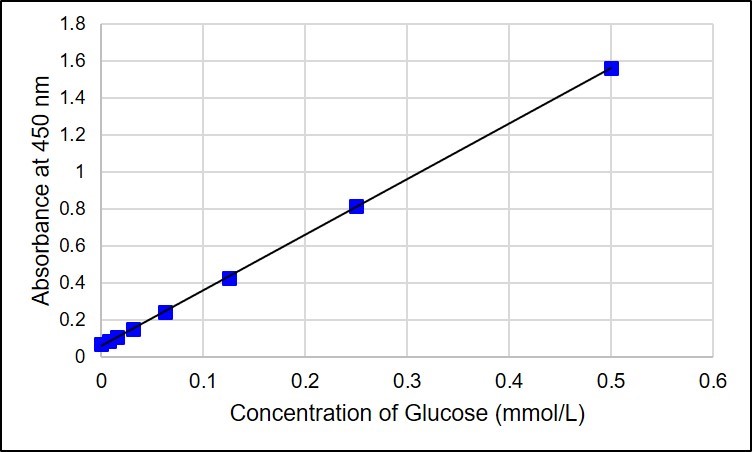
Explanation of the graph
Absorbance is directly proportional to the glucose concentrations. Therefore, the increase of absorbance leads to an increase in glucose concentrations. The parameters of the absorbance differ slightly with glucose concentrations.
Concentrations of glucose in A a, b, B a,b, C a, b
Concentrations = mass/volume
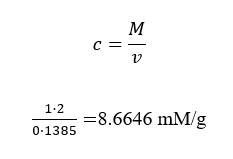
Concentration in B a, b
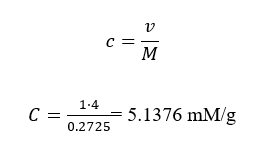
Concentration in C a, b
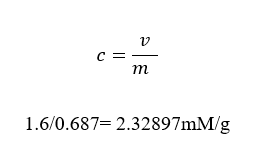
Content of glucose in A a, b, B a, b and C a, b
An a, b
Volume =mass/concentration
V= 100/1.2
=83.3333 mm
B a, b
Volume =mass/concentrations
V=100/1.4
=71.4286mm
C a, b
Volume = mass/concentration
V=100/1.6
=62.500mm
Pros of spectrophotometry
The biggest advantage of using a spectrometer is the accuracy and speed of the device. Most astronomers and chemists use the spectrometer to carry out their research that involves concentrations of unknown substances. Spectrometer UV-VIS can give very accurate readings, which is important when preparing chemical solutions in the lab. Most spectrometer devices are simple to use hence requires little skills.
Principles of Beer-Lambert law
The Beer Law relates the absorptions of UV light by the solution to the properties of the solution according to the following equations A= εbc. The ε is the molar absorptivity of absorbing types, b is the pathway length, and c is the absorbing species’ concentration.
Factors that cause deviation from Beer law
Deviations from the Beer-Lambert Law can be due to chemical reasons emanating from dissociations of compounds to produce a different absorption spectrum. Naturally, the Beer lambert law behaves correctly for concentrations below a critical point (Mendel et al.; 2016 ). When a Beer’s Law is subjected to real and apparent deviations, the unlimited variations are usually encountered in relatively concentrated solutions.
A calculated gram of glucose
The mass of glucose is always given by the concentration of the moles in the solution divided by the volume used. The higher the moles in the solution, the higher the mass of the glucose. The concentration of moles increases the dissociation rate of the reaction. The gram’s usefulness in the solution is that, after the respiration takes place, the glucose produces a high amount of energy used by the body cells.
Factors affecting accurate concentration in citrus fruits
Generally, accurate concentrations can be affected by several factors. In reducing sugars, the temperature is one of the factors that affect the concentration of glucose. An increase in temperature leads to a decrease in reaction since the enzymes acting on the glucose are denatured. Another factor affecting the concentration of the reducing sugars is the concentration of reactants. The increased glucose content of citrus fruits implicates increased reducing sugars.
Alternative methods to determine glucose concentration
Biologically, Benedict’s solution is the most used reagent for testing the presence of reducing sugar. When Benedict’s reagent’s blue solution is added into citrus fruits and heated, the color of the solution turns Yellow, thus indicating the presence of reducing sugar. The hotter the final color, the higher the concentration of glucose. Alternatively, reducing sugar can be tested using a burette test.
How GOD- PAP reacts with glucose
GOD-PAP reagent oxidizes the glucose such that glucose is oxidized into gluconic acid and hydrogen peroxide molecules react with PAP to form quinonimine. The specimen must be measured in 500nm for the absorbance colored complex and should be proportional to the glucose concentration. Alternatively, phenol’s color reaction to give quinonimine dyes of phenol and oxidizing reagents can be suggested basing measured wavelength.
Sources of errors in the experiment
In determining the glucose, accuracy can be limited because of band manufacturing variances and band storage of reagents used. The sources of errors can also come from surroundings such as high temperatures. High temperature may speed up the reaction since enzymes are active. The low temperature will slow down the rate of reactions since the enzymes become inactive.
Conclusion
In conclusion, the sweetness of citrus fruits like orange is due to several sugars’ presences. Sugars can be either invert sugar or reducing sugars. From the experiment, the citrus fruits contain glucose, which is crucial in the provision of energy. The experiment proves that glucose in the orange can be determined using several reagents, such as Benedict’s solution and GOD-PA. Besides these methods of determinations, their other suggested techniques can be applied.
References
Banauch, D., Brümmer, W., Ebeling, W., Metz, H., Rindfrey, H., Lang, H., … & Staudinger, H. J. (2017). A glucose dehydrogenase for the determination of glucose concentrations in body fluids (author’s transl). Zeitschrift fur klinische Chemie und klinische Biochemie, 13(3), 101-107.
Blank, T., Monfre, S., Makarewicz, M., Mattu, M., Hazen, K., & Henderson, J. (2005). U.S. Patent Application No. 10/841,200.
Dubowski, K. M.( 2015). An o-toluidine method for body-fluid glucose determination. Clinical chemistry, 8(3), 215-235.
Gochman, N., Ryan, W. T., Sterling, R. E., & Widdowson, G. M. (1975). Interlaboratory comparison of enzymatic methods for serum glucose determination. Clinical chemistry, 21(3), 356-361.
Keilin, D., & Hartree, E. F. (1948). The use of glucose oxidase (notatin) for the determination of glucose in biological material and the study of glucose-producing systems by manometric methods. Biochemical Journal, 42(2), 230.
Mendel, B., Kemp, A., & Myers, D. K. (2016). A colorimetric micro-method for the determination of glucose. Biochemical Journal, 56(4), 639.
Trinder, P. (2014). Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. Journal of clinical pathology, 22(2), 158-161.