Essay on Is This the End of Diabetes and Obesity?
Number of words: 1336
Hepatic secretion of glucose is vital for both the function and the survival of the brain, this is particularly important during periods of fasting and occurs in a process known as glycogenolysis. Asprosin is a glucogenic peptide hormone (restricted to mammals), it is released by white adipose tissue, which then trigger the liver to secrete glucose into the blood stream where it circulates at nanomolar levels (1). The hormone was first discovered at Baylor College of medicine by Dr. Atul Chopra (a medical geneticist) and colleagues (1).
This endogenous peptide is encoded by the gene FBN1, which is part of the protein profibrillin (located on chromosome 15) (1). It is released as a result of a particular proteolysis (cleavage) process, from the C-terminus of profibrillin (1). In regard to its endocrine actions (after release and circulation) it is recruited to the liver, here is activates the G protein-cAMP-PKA pathway and regulates the homeostasis of glucose (2). Although the specific mechanism of this hormone is yet to be discovered, it is thought that it uses a cell-surface receptor which is different to those specific for catecholamines and glucagon (3). Moreover, chemical reactions such as gluconeogenesis and glycogenolysis, occur due to hormones like glucagon, these processes work in a similar manner as asprosin (both involve cyclic AMP pathway in hepatocytes) (1).
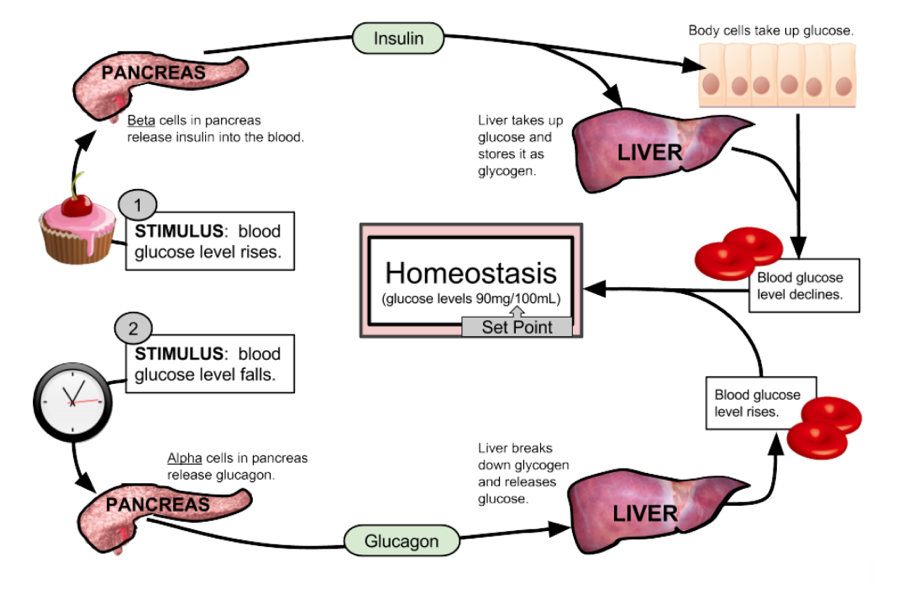
Figure 1- this diagram demonstrates glucose homeostasis by glucagon. When blood glucose levels drop, the alpha pancreatic cells release glucagon, this in turn allows the liver to break down glycogen into glucose and finally results in a rise in glucose levels (4).
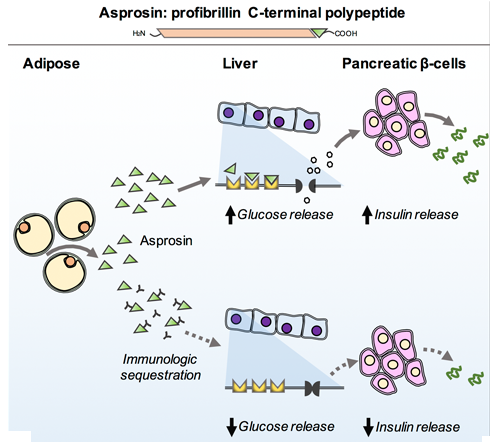
Figure 2- This diagram represents the effect of the hormone asprosin, it can be seen that it has a similar effect as glucagon in that it stimulates glucose release and the rise in glucose then causes insulin release. When asprosin is inhibited the levels of glucose release are decreased (5).
Regarding therapeutic potentials of asprosin, studies reveal that is has a possibility in treating type 2 diabetes (1). In a test conducted by Chopra, it was seen that when antibodies that targeted asprosin were given to mice with diabetes, both glucose and insulin levels in the blood improved (1).
Asprosin’s capabilities go far beyond the regulation of glucose levels, in 2017 Chopra identified that the hormone is able to cross the blood-brain barrier (BBB) and stimulate the ventromedial nuclei and lateral hypothalamus (which are the hunger centres of the hypothalamus) (5). This mechanism effectively is responsible for control of appetite and body weight (5). Asprosin is similar to ghrelin, in the sense that both are orexigenic hormones, however, ghrelin is secreted from epsilon cells in the stomach (6). The discovery of asprosin emerged from studying neonatal progeroid syndrome, which is an inherited form of lipodystrophy in humans (6). Individuals diagnosed with NPS often have metabolic problems and resistance to insulin (6). These are all considered to be secondary to insufficient adipose tissue. Asprosin levels in the blood increase throughout the night in fasting humans and mice and on the other hand the levels decreased following ingestion, this pattern reveals that asprosin has a role in signalling hunger (6).
The mechanism in which asprosin works is unique, as the hormone crosses the BBB it activates the orexigenic AgRP+ neurones and it does this through the cAMP-dependent pathway (7). This action consequently inhibits the anorexigenic proopiomelanocortin-positive neurones using GABA, this has a further effect of stimulating the appetite, thus collects fat and raises the weight (7).
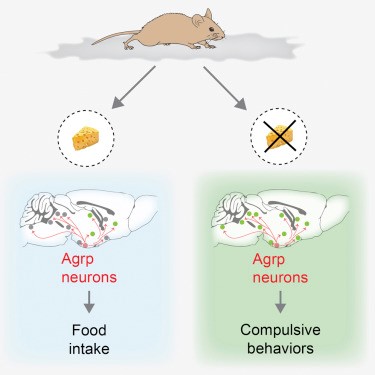
Figure 3- Pictorial description of asprosin and its effect on food intake (8).
In reference to the clinical significance of asprosin and its use as a treatment for obesity, by neutralizing asprosin in obese mice and humans (by using a monoclonal antibody) appetite and therefore food intake is reduced, this then ultimately leads to a loss of weight (7). Other than obesity, patients with insulin resistance and females that have polycystic ovarian syndrome (due to elevated androgens) also have high asprosin levels (1).
Fibrillin-1 ( a large glycoprotein coded by FBN1 gene is formed from the cleavage of profibrillin-1), is crucial for the formation of elastic fibres, any individual that possess a mutation in the FBN1 gene can be associated with conditions such as Marfan syndrome and autosomal dominant Weill-Marchesani syndrome (9)(1). Marfan syndrome is characterised by thin and long arms and legs (1). In addition to this, another condition known as Marfanoid-progeroid-lipodystrophy (which is quite similar to the Marfan syndrome and NPS) have mutations which directly affect the C-terminus (1). Therefore profibrillin-1 cannot be broken down into fibrillin-1 and asprosin (1). And so there is no asprosin hormone in those individuals.
The endocrine system (alongside the nervous system) are perhaps the most important players in the regulation and control of our bodily functions. Although each hormone circulates the entire body (in this case asprosin) it ultimately only affects one target organ (i.e. liver). It is necessary that the system alongside its hormone work as many diseases may arise, for example, diabetes which is said to be one of the most common endocrine diseases in the United States.
The discovery of the hormone asprosin has been both an important and vital movement in the progression of the scientific and medical field. It shows that there is still fundamental uncovering of hormones to be made and is a clear example of our bodies own clever machinery, design and adaptation to fight certain conditions. For example, asprosin is an endogenous hormone that we can manipulate to treat some of the world’s most serious diseases like diabetes-2 and obesity. We are now able to manipulate the hormone using genetics to better suit those affected by the complications, this not only allows control of the illness but also increases the life span. And although our scientific knowledge of this hormone has only gone up to its role in improving the glycemic profile and acting as an orexigenic hormone, it is fair to assume its uses will expand (7). The work conducted by Dr.Chopra and his findings about the new adipokine has been revolutionary, and despite the fact that the entire mechanisms in which this hormone operates in has not yet been fully uncovered, its potential in restoring health in many patients is a possibility and marks as an important event in the evolutional of science.
Bibliography
- contributors W. CiteThisPage [Internet]. En.wikipedia.org. 2019 [cited 29 October 2019]. Available from: https://en.wikipedia.org/w/index.php?title=Special:CiteThisPage&page=Asprosin&id=918132199
- romere c. Asprosin, a Fasting-Induced Glucogenic Protein Hormone [Internet]. Pubmed. 2016 [cited 30 October 2019]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4852710/
- asprosin | Ligand page | IUPHAR/BPS Guide to PHARMACOLOGY [Internet]. Guidetopharmacology.org. 2019 [cited 30 October 2019]. Available from: https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9200
- Muskopf S. Feedback Loops: Glucose and Glucagon [Internet]. Biology LibreTexts. 2019 [cited 30 October 2019]. Available from: https://bio.libretexts.org/Ancillary_Materials/Worksheets/Book%3A_The_Biology_Corner_(Worksheets)/Anatomy_Worksheets/Feedback_Loops%3A_Glucose_and_Glucagon
- Rodríguez A. The multi-tasking nature of the hormone asprosin [Internet]. Baylor College of Medicine Blog Network. 2019 [cited 30 October 2019]. Available from: https://blogs.bcm.edu/2018/01/19/the-multi-tasking-nature-of-the-hormone-asprosin/
- Beutler L. A spotlight on appetite [Internet]. Pubmed. 2018 [cited 1 November 2019]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5965268/
- Duerrschmid C. Asprosin is a centrally acting orexigenic hormone [Internet]. Pubmed. 2017 [cited 1 November 2019]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/29106398
- Dietrich M. Hypothalamic Agrp Neurons Drive Stereotypic Behaviors beyond Feeding [Internet]. cell.com. 2015 [cited 1 November 2019]. Available from: https://www.cell.com/cell/fulltext/S0092-8674(15)00190-7
- Davis M. Expression of FBN1 during adipogenesis: Relevance to the lipodystrophy phenotype in Marfan syndrome and related conditions [Internet]. Pubmed. 2019 [cited 1 November 2019]. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5044862/