How glutathione acts to protect mammalian organisms from potentially toxic exogenous and endogenous compounds
Structure and Role in Detoxification
One of the fundamental molecules mammalian organisms exploit at the cellular level to protect against potentially toxic exogenous and endogenous compounds is glutathione (γ- glutamyl-cysteinyl-glycine, or GSH). It is an abundant tripeptide primarily concentrated in the liver at levels of 5mM or more, though it can be found in most mammalian cells within the range of 0.5 – 10mM (Timbrell, 2009 and Wu et al., 2004). The structure of the molecule is recognisable by its glutamic acid, cysteine and glycine residues (Fig. 1).
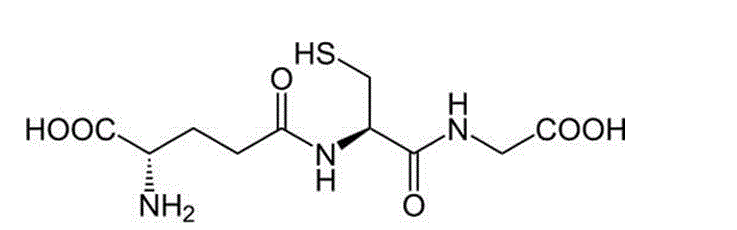
Figure 1: Chemical structure of Glutathione [source: http://chemistry.about.com/od/factsstructures/ig/Chemical-Structures—G/Glutathione.htm Accessed: 10 Oct 2012]
The presence of the reactive sulphydryl group (originating from the cysteine residue) gives rise to GSH’s nucleophilic nature and so it reacts readily with electrophiles which can present high risk of toxicity to the cell by causing oxidative damage such as lipid peroxidation to cell membranes, and interfering with the activity of essential proteins and DNA synthesis. Simply put – the reactions of glutathione help to remove potentially toxic and reactive metabolites from cells. Glutathione is subsequently found at its highest concentrations in tissues at most risk and therefore requiring the most protection from potentially toxic exogenous and endogenous compounds: the liver – where the vast majority of detoxification metabolism occurs – and, at the cellular level, around organelles which are most exposed and most at risk of potential damage from oxidative stress from reactive oxygen species: mitochondria, peroxisomes and the nuclear matrix (Wu et al., 2004).
Major Antioxidant: Synthesis and Mechanisms of Action
Timbrell, 2009, lists the three principle mechanisms of detoxification with glutathione:
1) Conjugation chemical reaction with glutathione
2) Conjugation catalysed by GSH transferase enzymes
3) Reduction by the donation of a hydrogen atom or proton as a result of oxidation of GSH.
Glutathione conjugation is one of the major metabolism reactions which occur as part of “Phase II” metabolism in the liver. Phase I metabolism, as a general rule, generates very reactive oxidising compounds. GSH undergoes conjugation reactions with these very reactive compounds – generally rendering metabolites far less oxidising and reactive and more polar and hydrophilic than their parent compounds and so easier to excrete. The major role glutathione plays as a protective molecule is by shielding mammalian cells from unwanted oxidative damage. Though this can be sufficient to detoxify some substances, some compounds that undergo glutathione conjugation (such as naphthalene) require further metabolism for safe excretion. Sometimes termed Phase III reactions, these usually consist of the removal of glutamyl and glycinyl groups and acetylation of cysteine to produce mercapturic acid or an N-acetylcysteine conjugate which can then be excreted, usually via bile or urine (Figure 2).
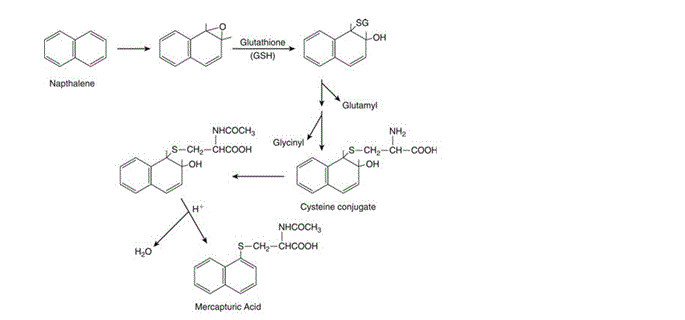
Figure 2: “Conjugation of naphthalene-1,2-oxide with glutathione and formation of naphthalene mercapturic acid” [Figure source: Timbrell (2009) Biochemical Mechanisms of Toxicity.4th Ed. New York: Informa Healthcare]
Figure 3 illustrates how glutathione is recycled for use in detoxification reactions. Glutathione can act as a reducing agent – mediated by an enzyme or otherwise – in an oxidation reaction which leads to the direct conversion of GSH to its oxidised form of GSSG. The examples described in the figure are those describing oxidation of GSH mediated by GSH peroxidase and thiol transferase. Proton donation by GSH to form a free radical is also described though it differs in that the free radical formed can pair with another GSH radical to produce GSSG or extract a hydrogen radical to form new radicals and reform GSH. Upon production of GSSG, the molecule undergoes a reduction reaction dependent on NADPH reductase – which transfers a hydrogen atom from NADPH to GSSG to reform GSH and NADP+. γ glutamylcysteine synthetase is the enzyme which catalyses the synthesis of new GSH and maintains the pool of glutathione used by the cell for conjugation of toxic compounds. Synthesis of GSH can be limited by the amount of cysteine, glutamate and glycine available in the cell, while recycling of GSSG can be limited by the amount of NADPH available for reduction. If the cell’s supply of glutathione is severely depleted and GSSG trafficking out of the cell exceeds the rate at which it can be reduced back to GSH, cellular defence against oxidative stress is impaired and the cell may experience critical membrane failure and even cell death as a result of lipid peroxidation due to lack of protective glutathione antioxidant action.
Glutathione also participates in other cellular defences against toxicity. Washburn and Wells, 1999, describe the activity of a glutathione-dependent dehydroascorbate reductase which is able to regenerate the potent antioxidant, ascorbate, from its oxidated product by using glutathione as a reducing agent and electron donor. In a more recent paper, James et al., 2005, describes how glutathione provides one of the most important intracellular defences against mercury-induced neurotoxicity. These examples illustrate that although glutathione is a potent antioxidant by itself, it also works protectively with other mechanisms to protect the cell from harm.
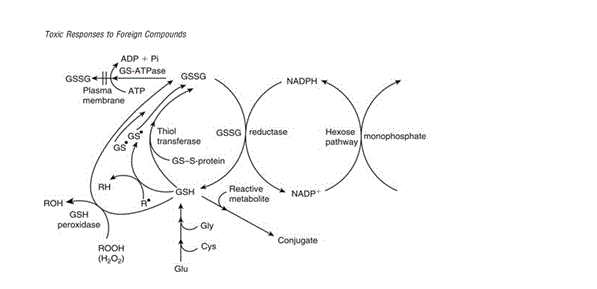
Figure 3: “The various protective roles of reduced GSH and the relationship with GSSG. NADPH may be regenerated by the pentose phosphate shunt.” [Figure source: Timbrell (2009) Biochemical Mechanisms of Toxicity.4th Ed. New York: Informa Healthcare]
GS-S transferases in Cancer Susceptibility
Substrates subject to glutathione conjugation share specificity for the tripeptide in that most are hydrophobic; are able to react with glutathione without the presence of a catalytic enzyme and contain an electrophilic carbon atom at which glutathione conjugation is targeted. Despite these shared traits, GS-S transferases coded for by the same genotype can have differing (though occasionally overlapping) substrate specificity and activity depending on the tissue they are found in and there is much research trying to discover what effect these genetic differences may have on an individual’s idiosyncratic response to certain substances such as carcinogens. Research is questioning whether it is possible to identify GS-S transferases which would be phenotypically more or less active in resisting a substance’s effect and why this would be the case (Coles & Kadlubar, 2003). Thus, glutathione can be considered to play a sometimes-protective role in susceptibility to cancer.
Protein and cell regulation
GSH has been shown to interact and bind with a variety of membrane and cytosolic proteins: modulating their function and affecting signal transduction chains. Pompella et al, 2003 describe a number of signalling cascades affected by “protein S-glutathionylation”: NF-kB/p50 (Pineda-Molina et al., 2001) and PTP1B phosphatase (Barrett et al., 1999), for example, which are able to affect cell proliferation. Thus, glutathione plays not only a role in protein regulation, but also in regulation of the cell cycle and programmed cell-death (apoptosis). Franco and Cidlowski, 2009, describe how new research suggests that glutathione depletion, particularly in the mitochondria, and glutathionylation modification of proteins after translation may play a significant part of the regulation of apoptosis, and seem to pre-empt it rather than just being by-products of the process itself.
Toxic Conjugants of Glutathione
Though glutathione plays a mostly protective role in the mammalian cell, it is important to acknowledge that it can also play a role in increasing toxicity as well as preventing it. An example is 1,2-dibromoethane: a dihaloalkane which forms a toxic conjugant after catalysis with a GS-S transferase. Loss of both bromine ions is followed by the formation of a highly reactive episulfonium ion which acts as a DNA adduct which is believed to be the root of the compound’s tumourigenicity and mutagenicity (Figure 4).
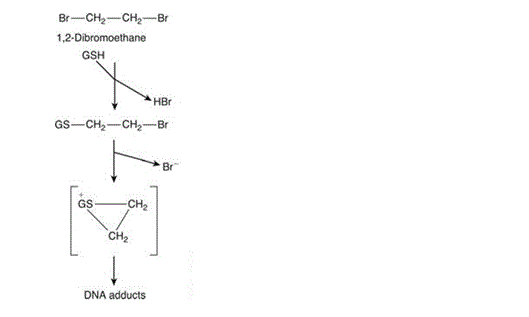
Figure 4: Glutathione conjugation with 1,2-dibromoethane causing formation of the highly reactive episulphonium ion [Figure source: Timbrell (2009) Biochemical Mechanisms of Toxicity.4th Ed. New York: Informa Healthcare]
Conclusion
Glutathione is a well-known protective molecule in mammalian organisms and plays a particularly strong antioxidant role in the cell. The main mechanism of GSH-GSSG cycling by which this occurs is quite well understood. Though this is perhaps one of its most prominent and perhaps obvious functions, research in recent years has shed light on the potential other functions of glutathione that are perhaps equally as important in cell maintenance and regulating controlled cell death, with implications for tendency to develop cancer. Finally, as much as it is important to shed light on the potential “good” that glutathione performs as a protective molecule, it is equally important to discover and observe in which ways it is certainly not protective and what elements of a compound-conjugate can trigger its toxicity instead.
References
Barrett, W.C., DeGnore, J.P. and Konig, S. et al. (1999) Regulation of PTP1B via gltathionylation of the active site cysteine 215. Biochemistry, 38(20): 6699-6705
Coles, B. and Kadlubar, F. (2003) Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinanats of individual response to chemical carcinogens and chemotherapeutic drugs? Biofactors, 17(1-4): 115-130
Franco, R. and Cidlowski, J.A. (2009) Apoptosis and glutathione: beyond an antioxidant. Cell Death & Differentiation, 16(10): 1303-1314
James, S.J., Slikker, W. 3rd. and Melnyk, S. et al. (2005) Thimerosal neurotoxicity is associated with glutathione depletion: protection with glutathione precursors. Neurotoxicology, 26(1): 1-8
Pineda-Molina, E., Klatt, P. and Vasquez, J. et al. (2001) Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry, 40: 14134-14142
Pompella, A., Visvikis, A. and Paolicchi, A. et al. (2003) The changing faces of glutathione, a cellular protagonist. Biochemical Pharmacology, 66 (8): 1499-1503
Timbrell, J. (2009) Principles of Biochemical Toxicology. 4th Ed. New York: Informa Healthcare
Washburn, M. P. and Wells, W. W. (1999) The catalytic mechanism of the glutathione-dependent dehydroascorbate reductase activity of thioltransferase (glutaredoxin). Biochemistry, 38: 268-274
Wu, G., Fang, Y.Z. and Yang, S. et al. (2004) Glutathione metabolism and its implications for health. The Journal of Nutrition, 134 (3): 489-492