Is Clostridium difficile under-diagnosed in the community?
Background
Clostridium difficile infection (CDI) causes mild to severe diarrhoea which can lead to life threatening conditions such as pseudomembraneous colitis. Clostridium difficile infection is generally characterised by prior hospitalisation, prior antibiotics usage, older age and co-morbidities. Clostridium difficile causes outbreaks in hospital which maybe as a result of the community being a reservoir for the bacterium. The incidence of community acquired Clostridium difficile (cCDI) is unknown and largely under diagnosed. There is a growing number of CDI in the community in previously healthy people and peripartum women, without any hospitalisation or antibiotic usage. Method: The study will use a descriptive method followed by an unmatched case control method to identify new risk factors contributing to CDI, apart from the classic risks. Participants will include those treated in the London Borough of Newham, and who have been diagnosed as positive for either both hospital acquired Clostridium difficile or community acquired difficile, and a control group who tested negative. Both groups will be compared to controls which have tested negative for the disease. As well as this, questionnaires will be sent out to GPs around the Newham Borough of London via the safe NHS. net to establish the diagnosis attitudes of the GPs. Result: A statistical matched analysis and applied regression model will be used to analyse data. Responses to the questionnaire will be a subject to descriptive analysis.
INTRODUCTION
Clostridium difficile (C difficile) is an anaerobic, gram-positive, spore forming bacillus capable of producing significant diarrhoea or colitis (Efron and Mazuski, 2009), especially in those exposed to antibiotics in recent weeks prior to onset of diarrhoea. Clostridium difficile is a common pathogen associated with infectious diarrhoea in hospitalised patients, and high cases are seen in surgical patients due to the range of prophylactic treatment required prior to surgery (Efron and Mazuski, 2009).
In recent years C difficile infections have been increasing in incidence and severity, partly due to the rise of a pathogenic strain, the BI/NAP1/027 strain (Efron & Mazuski, 2009). C difficile strains that produce exotoxins, namely A and B, are pathogenic. Toxin B is 10 times more potent than toxin A (Efron and Mazuski, 2009). The strain known as “BI/NAP1/027” has recently been associated with producing severe symptoms. This strain produces more of toxins A and B than the other strain, causing a more severe form of diarrhoea (Riley, 2009). Toxin A is responsible for the deletion of strains tcdC gene, which is a negative regulator of toxin producer (Efron and Mazuski, 2009), and causes production of both toxin A and B to increase by 16-23 folds (Efron and Mazuski, 2009). The strain is also resistant to fluroquinoloes, and has hypersporulation capacity (Efron and Mazuski, 2009), producing severe toxins leading to diarrhoeal attacks.
The common risk factors associated with C difficile are exposure to antibiotics, patients being hospitalised or having contact with the healthcare services commonly, and the elderly with a depleted immune system, allowing the organism to proliferate (Efron and Mazuski, 2009).
Increased exposure to broad spectrum antibiotics increases the risk of developing C. difficile infection up to six folds or more, with the most common antibiotics which predisposes are those broad spectrum antibiotic such as clindamycin, cephalosporins, some penicillins (Efron and Mazuski, 2009). In recent studies, fluoroquinolones have been implicated in the cause of C difficile infection. The exposure to healthcare settings tends to be secondary in increasing the risk of acquiring CDAD (Efron and Mazuski, 2009).
Community acquired CDI attack rate of community acquired in antimicrobial associated colitis and requiring hospitalisation was 1.4 per 100,000 populations (Kutty et al., 2010). In a study by Kutty et al. (2010) of 580 CDI toxin positive stools samples submitted from patients with diarrhoea and a clear history of recent (< 4 weeks) antimicrobial exposure, 10.7 were from patients who did not have a recent history of hospitalisation. In addition the incidence from 7.7-12.2 cases of CA-CDI per 100,000 was recorded in the US communities to 25 per 100, 000 people in Sweden (Kutty et al., 2010).
According to the HPA Quarterly Epidemiology Commentary data published in June 2010, the secondary care trust apportioned CDI has decreased in the number of cases by 14% from January – March 2010 (3,485 to 2,996), however there has been a 4% increase in non-trust apportioned (community acquired) (2,856 to 2,987).
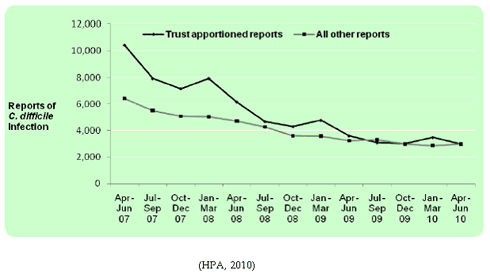
Figure 5: Counts of Trust apportioned and all other reports of clostridium difficile infection, April – June 2007 to April – June 2010
It is known that Clostridium difficile can cause major infectious diarrhoea, which can have fatal consequences, especially to those who are immuno-suppressed or elderly (Fellmeth et al., 2010). It is predominately known as a disease that affects the elderly but in recent years more studies have suggested there are many which asymptomatic carriers of the disease. It is also apparent that many who suffer from CDI have not been exposed to antibiotics and yet show symptoms (Fellmeth et al., 2010).
Methodology
A case-control method will be implemented, which will examine and compare the characteristics between individuals with hospital acquired CDI, community acquired CDI and those who tested negative (control). A descriptive analysis of questionnaire response and data from HPA mandatory surveillance will be statistically analysed for assessing any casual causation for C. difficile infection.
The method will follow a similar principle to Kutty, P., et al. (2006) in their study to estimate the incidence of and the risk factor for community associated Clostridium difficile amongt six North Carolina hospitals. Their cases included reviewing C. difficile toxin assays from the local infection control database; computer patient records systems (Kutty et al., 2006) and the surveillance database of the Duke University Hospital network. as Also, a retrospective analysis of dedical and laboratory records were reviewed for case patients (Kutty et al., 2006).
Their case definitions included patients defined as a person >18 years of age with a non-formed stool specimen with positive test result for C. difficile toxin (Kutty et al., 2006). Patients that had a positive test result in the 8 weeks preceding collection were excluded. Community acquired c diff were defined as:
1. in an outpatient setting.
2. < 3 days calendar after hospital admission.
Risk of case-control
The case control method will provide data from which the rate of exposure to suspected harmful agents in diseased and non-diseased individuals can be calculated (Farmer and Lawrenson, 2004). This means that neither the absolute risk, the attributable risk or a precise relative risk resulting from exposure can be calculated (Farmer and Lawrenson, 2004). This approximation is referred as relative risk, or odds ratio (Farmer and Lawrenson, 2004).
This approximation to relative risk is valid only if the incidence of the disease is low. Similarly this case-control method will anticipate confounding variables that will need to be taken into account (Farmer and Lawrenson, 2004). A multivariate analysis technique will be used to adjust the risk for the effect of confounding variables (Farmer and Lawrenson, 2004).
An unmatched case controlled will be conducted in contrast to Dial, S., et al. (2008) and used in their study for assessing the antibiotic use and risk of hospital admission because of Clostridium difficile infection. Dial, S., et al. (2008) study used a matched nested case control study amongt the cohort of all elderly people in the province of Quebec. This included all those aged 65 or above and only included patients who had at least one hospital stay during the study period. They excluded any C diff diagnosis as a secondary case or relapses.
The advantage of this method was that by restricting the population they controlled the factors associated with hospital admission and controlled confounding factors by the severity of illness by matching (Dial et al., 2008). They excluded cases previously testing positive for C. diff, which reduced the bias of antibiotic estimates, as with those who previously tested positive, the physician would be less likely to use antibiotics as they would be of high risk to patient (Dial et al., 2008).
One of the major limitations of the study was it only included the elderly population who were admitted to the hospital in the previous 6 years at least once (Dial et al., 2008). This limited the representation of the results to the wider population as the patients studied were not well selected.
Also the case definition used in their study was restricted to severe infection and therefore the risk factor observed in the normal population would differ. I would also look to assess younger healthier individuals who may be reservoirs.
This is the reason for ensuring this research would use an unmatched case control method as it will provide means to assess all confounding factors in all age groups of positive results to establish any relationship between developing of C. difficile, as well as identifying the younger healthier patients harboring ClostridiumClostridium difficile.
Cross sectional
A cross sectional survey will be emailed to selected GPs within the Newham Borough of London. The advantage is that the survey can be very large and spread over a wide geographical area. The spread brings little or no extra cost as a single price of a postage stamp and brings regional coverage. The survey will require minimal effort from the participants, as they will not need to decline participation in a face-to-face situation, but the obvious disadvantage is that they can refuse to comply merely by ignoring the request (Farmer and Lawrenson, 2004). They can also partially comply by failing to follow instruction on the questionnaire, or by omitting responses to those questions that are difficult or perhaps embarrassing to complete. It must be anticipated that a low response rate is a real possibility (Farmer and Lawrenson, 2004). In addition, postal surveys tend to have a lower response rate from certain specific groups.
Sampling of choice: Systemic Sampling
Systematic Sampling
The target population is the East London Borough of Newham, and its GPs and other outpatients doctors will be targeted on their knowledge of diagnosing Clostridium difficile. Newham Borough is known as one of the most diverse areas of England due to the cultural population in the borough and will represent whole of London.
There will be a systematic sampling, as GPs are selected at regular intervals from a list of all GPs covering the area. This form is convenient and adequate for this purpose. Bias in the sampling will be inevitable to an extent including large number of individuals in the sample refusing to co-operate in the study (Machin and Campbell, 2005), therefore the result maybe meaningless. Similarly the list of GPs in the community maybe out of date, bias maybe introduced owing to omission of recently registered GP and no responses from those who have left.
Collection of data from cases and controls
Data will be available through Newham University Hospital Medical Records and pre-existing records. In addition a questionnaire will be sent to GPs in Newham PCT to assess their medical knowledge of accurate diagnosis and whether their diagnosis plays any part in the under-diagnosed Clostridium difficle in the community, leading to exposure to the organism.
The HPA Data Capture Website and UK General Practice Register will provide raw data on community acquired and hospital acquired Clostridium difficile, antibiotic prescribing in the community, and I will use Newham University Hospital patients as part of my case control study.
The use of questionnaires will highlight if there is any lack of clarity in diagnosing ClostridiumClostridium difficile, as Barbut, F.,et al. (2003) found in their study, which included conducting a survey to assess the method used in clinical microbiology laboratories in Europe to diagnose infection with C difficile infection. The method introduced was a standardised questionnaire about the diagnostic methods for Clostridium difficile, which was sent to eight participating countries. The survey covers aspects such as request, criteria and methods for diagnosing Clostridium difficile.
The questionnaires returned and analysed covered a total of 189,682 beds from all countries that participated (Barbut et al., 2003). The data collected helped to estimate the average incidence of CDAD across participating European countries as 1.1 per 1000 patient admissions (Barbut et al., 2003). This approach does provide a large amount of data that can be representative of countries, as well as providing statistical analysis (Barbut et al., 2003).
However, limitations in their study included the selection of laboratories and those contacted will be those perceived to respond to questionnaire.
The methods have an excellent advantage in obtaining vast amount of data, which can be analysed for generalisation (Polgar and Thomas, 2000).
Nominal scales will be introduced where the measurements of a variable involve the naming or categorisation of possible values (Polgar and Thomas, 2000). These measurements will be qualitative and numbers are assigned.
Statistical analysis
Correlation
Correlation coefficient will be used, which expresses findings numerically, , providing a visual representation of relationship between two variables (Polgar and Thomas, 2000).
Alfa, M., et al. (1998) used a correlation statistics in their study of the incidence of C difficile in Canadian hospital which helped to assess the correlation between hospital size and disease incidence. In this particular study a correlation will be used to assess what risk factors predispose of Clostridium difficile.
A correctional coefficient will be used in this assignment to assess whether criteria for assessing Clostridium difficile between GPs are a factor in acquiring C. difficile (Wilcox et al., 2008).
Similarly this study will use a multivariate analysis to assess any risk between factors. This was a procedure which Dias, S., et al. (2008) used in their eight-year study, in which they had a total of 836 cases, from which 394 (47.1%) had been exposed to antibiotics in the 45 day period before the index admission. On multivariate analysis, all antibiotic classes other than the tetracycline, trimethoprim-sulfamethozole and antibiotics classified as “other” were associated with increased risk, with the highest being clindamycin and cephalosporins and one of the quinoloines (gatifloxacin).
The study will look to estimate the odds ratio as an approximate of the rate ratio of CDAD for the risk factors related in this study. This will follow similar method to Dial, S., et al. (2008) study for gastric acid and suppressants and Clostridium difficile,which included analysing data by the use of logistic regression. This helped to estimate the odds ratio CDAD.
In their first analysis, their entire series of cases and their controls, RRs of CDAD were estimated for age, sex, hospitalisation in the year prior to the index case and current antibiotic exposure.
In their second analysis, rate ratio for community acquired CDAD were estimated for current use of proton pump inhibitors, H2RAs, non-steroidal anti-inflammatory drugs (NSAIDs), aspirin, and antibiotics after adjustment for sex co-morbidity and co-prescription with NSAIDs and aspirin (Dial et al., 2008). All analysis was performed using SAS statistical software version 8.2 and p<.05 after Bonferroni correction was considered significant (Dial et al., 2008).
Aim of project
Clostridium difficile outbreaks have occurred in a range of hospitals and continue to do so. It costs lives and most causes are avoidable. This project will look to assess how widespread the organism is in the community and asess how this will affect the health of the patient later in on life.
Aim: “To evaluate community acquired Clostridium difficile infections in patients with diarrhea, who have no known risk factors”.
There needs to be better diagnostic criterion for identifying potential risk factors for community acquired Clostridium difficile, as large amounts of evidence show a large proportion do not need to have recent exposure to antibiotics or a hospital stay.
The objectives of the studies are:
- Establish the prevalence and incidence of Clostridium difficile in the Community of Newham, representing the East London community.
- Assess the diagnostic criteria and whether it’s suitable to diagnose Clostridium difficile in the community.Medical practitioners should not rely on the standard signs and symptoms in diagnosing Clostridium difficile infection, including the use of recent antibiotic use, prior hospital stay, co-morbidity and being over the age of 65 years old.
- Critically analyse whether other factors apart from broad spectrum antibiotics predispose the risk of acquiring Clostridium difficile, including the use of Proton Pump Inhibitors (PPI).
- Assess the mode transfer of the organism in the community.
References
Care Quality Commissions (2006) Investigation into the outbreak of Clostridium difficile at Stoke Madeville Hospital, Buckinghamshire Hospitals NHS Trust. Available at:
http://www.cqc.org.uk/_db/_documents/Stoke_Mandeville.pdf. (Accessed: 14 November 2010).
E, Kuijper. & J, Van Dissel. (2008) ‘Spectrum if Clostridium difficile infections outside health care facilities’, The Canadian Medical Association Journal, 179 (8), pp. 747-745.
F, Barbut., M, Delmee., J, Brazier., J, Petit., J, Poxton., V, Lalande., C, Schneider., P, Mastranonio., R, Alonso., and M, Tvede. (2003) ‘ A European survey if diagnostic methods and testing protocols for Clostridium difficile’, Clinical Microbiology and Infection, 9(1), pp. 989-996.
Farmer, R. & Lawrenson, R. (2004) Epidemiology and Public Health Medicine. 5th edn. Oxford: Blackwell Publishing Ltd.
G, Fellmeth., S, Yarlagadda., and S, Lyer. (2010) ‘Epidemiology of community-onset Clostridium difficile infection in a community in the South of England’, Journal of Infection and Public Health, 3, pp. 118-123.
H, Pituch. (2009) ‘Clostridium difficile is no longer just a nosocomial infection or an onfection of adults’, International Journal of Antimicribial Agents, 33(1), pp.42-45 Elsevier [Online].
www.elsevier.com/locate/ijantimicag. (Accessed: 28 November 2010).
Health Protection Agency (2010) Quarterly Epidemiology Commentary: Mandatory MRSA Bactermaeia & Clostridrium difficile infection (up to April – June 10). Available at:
www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1287146910738. (Accessed: 16 November 2010).
J, Linesky., C, Pothoulakis., S, keates., M, Warny., A, Keates., J, Lamont., and C, Kelly. (1997) ‘IL-8 release and neutrohil activation by Clostridium difficile toxin-exposed human monocytes’, American Physiology Society, 273(6), pp. 1333-1340.
K, Roberts. (2005) ‘ Management of Clostridium difficile’, Nurse to Nurse, 5(1), pp. 38-41.
L, Beaugerie., A, Flahault., P, Atlan., V, Lanade., P, Cousin., M, Cadilhac., and J, Petit. (2003) ‘Antibiotic-associated diarrhea and Clostridium difficile in the community’, Alimentarty Pharmacology & Therapeutics, 17(7), pp 905-912.
M, Alfa., T, Du., and G. Beda. (1998) ‘Survey of Incidence of Clostridium difficile Infection on Canadian Hospitals and Diagnostic Approaches’, Journal of Clinical Microbiology, 36(7), pp. 2076-2080.
M, Bauer., A. Goorhuis., T, Koster., S, Numan-Ruberg., E, Hagen., S, Debast., E, Kuiper., and J, Van Dissel. (2008) ‘Community-onset Clostridium difficile-associated diarrhea not associated with antibiotic usage’, The Journal of Medicine, 66(5), pp. 207-211.
M, Wilcox., L, Mooney., R, C, Settle, and W, Fawley. (2008) ‘A case-control study of community-associated Clostridium difficile infection’, Journal of Antimicrobial Chemotherapy, 62, pp. 388-396.
Machin, D. & Campbell, M. (2005) Medical Research. 1st edn. Chichester: John Wiley & Sons Ltd.
P, Efron & J, Mazuski. (2009) ‘Clostridium difficile colitis’, The American Journal of Surgery, 89, pp. 283-500 Elsevier [Online]. Available at:
http://linkinghub.elsevier.com/retrieve/pii/S0039610908001485. (Accessed: 12 December 2010).
P, Kutty., C, Woods., A, Sena., S, Benoit., S, Naggie., J, Frederick., S, Evans., J, Engel., and C, McDonald. (2006) ‘Risk factors for and Estimated Incidence of Community-associated Clostridium difficile Infection, North Carolina, USA’, Emerging Infectious Diseases Journal, 16(2)197-203.
Polgar, S. & Thomas, S. (2000) Introduction to Research in the Health Sciences. 4th edn. London: Churchill Livingstone.
R, Owens. (2007) ‘Clostridium difficile-Associated Disease Changing Epidemiology and Implications for Management’, Drugs, 67(4), pp. 487-502.
S, Dial., A, Kezouh., A, Dascal., A, Barkun., and S, Suissa. (2008) ‘Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection’, Canadian Medical Association Journal, 179(8), pp. 767-771.
S. Dial, J, Delaney., A, Barkun., and S. Suissa. (2008) ‘Use of Gastric Acid-Suppressive Agents and the Risk of Community-Aquired Clostridium difficile-Associated Disease, Journal of the American Medical Association, 294(23), pp. 2989-2995.
T, Riley. (2009) ‘Is Clostridium difficile a threat to Australis’s biosecurty?’, The Medical Jounral of Australia, 190(12), pp. 661-662.
Bibliography
B, Limbago., C, Long., A, Thompson., G, Killgore., G, Hannett., N, Havill., S, Mickelson., S, Lathrop., T, Jones., M, Park., K, Harrimann., L, Gould., L, McDonald., and F, Angulo. (2009) ‘Clostridium difficile Strains from Community-Associated Infections’, Journal of Clinical Microbiology, 47(9), pp. 3004-3007.
Gerstman, B. (1990) Epidemiology Ketp Simple. 1st edn. Danvers: Wiley-Liss.
G, Terhes., E, Urban., J, Soki., K, Hamid., and E, Nagy. (2004) ‘Community-Aquired Clostridium difficile Diarrhea Caused bu Binary Toxin, Toxin A, and Tozin B Gene-Positive Isolates in Hungary’, Journal of Clinical Microbiology, 42 (9), pp. 4316-4318.
M, Branning. (2009) ‘Understanind the microbiology, prevalence and pathology of Clostridium difficile’, Gastroinestnal Nursing, 5(10), pp. 34-39.
M, Hooker. (2007) ‘Clostridrium Difficile’, Clinical Journal of Oncology Nursing, 11(6), pp. 801-804.
MMWR. (2008) ‘Surveillance for community Associate Clostridium difficile – Connecticut, 2006’, Center for Diseases and Prevention, 4 April 2008.
S, Kolli, A, Mallipedhi., R, Thomas., and M. Reddy. (2009) ‘Injudicious antibiotic use leading to fulminating Clostridium difficile infection: a case report’, Cases Journal, 2 (7978), pp. 1-3 Cases Journal Open Access [Online]. Available at:
http://casesjournal.com/casesjournal/article/view/7978.