Treatments of Multiple Myeloma and Associated Bone Disease.
Introduction
Multiple myeloma is a type of incurable plasma cells malignancy. This disease is characterized by clonal expansion of myeloma cells in the bone marrow, causing bone annihilation, impaired haematopoiesis, renal failures and other bone pathological fractures resulting in bone metastases. In the UK, nearly 3,300 new cases per annum are multiple myeloma(1).
In healthy adults, the adult skeleton bone is removed by large multinucleated cells of monocyte-macrophage cells, namely osteoclasts and this is followed by new bone synthesis by bone-forming cells of mesenchymal lineage cells, namely osteoblasts. This process is called ‘continual replacement’ in the healthy adult’s skeleton. The two processes are balanced in healthy adults, whereas in the case of pathological states such as cancer metastasis of skeleton, the process equilibrium is disturbed. Multiple myeloma is osteolytic; it distresses the skeleton and results in painful lytic lesions which may predispose to bone pathological fractures, decreased bone mineral density and hypercalcemia(2).
In addition, bone resorption is the key process for bone metastasis and the consequent development of secondary lytic lesions. Considering the pathway of bone resorption may afford innovative insights into the machinery of bone metastases and result in the development of novel approaches for the prevention and treatment of bone metastasis(2).
Regulation of bone resorption by OPG/RANKL/ RANK system
The identification of the OPG/RANKL/RANK system as a dominant, ultimate mediator of osteoclastogenesis is a major step forward in bone biology. Under normal and pathological condition such as cancer metastasis of the skeleton, osteoclasts are accountable for bone resorption. In turn, osteoclast formation is regulated by osteoblasts and other mesenchymally derived cells like bone marrow stromal cells(3).
The Amgen Group revealed that osteoporosis is related with a profound decrease in osteoclastogenesis and osteoclast activation. Furthermore, they revealed that major negative regulators of bone resorption are OPG (osteoprotegerin) molecules which play a key role in bone protection and regulating osteclastogenesis. Soon thereafter, the Amgen Group, using OPG as probe, identified the OPG ligand molecule/osteoclast differentiating factor and receptor activator of nuclear factor kappa B ligand (RANKL) molecules as an important mediator of osteoclastogenesis(4).
The molecular triad, namely OPG, RANK, RANKL, is produced by various types of cells and different tissues like osteoblasts, endothelial cells, smooth muscle cells, dendritic cells and lymphocytes. Although the molecular triad is involved in the orchestration of patho-physiological bone remodelling, other molecules like tumour necrosis factor alpha and Interleuckin-1 are involved in controlling the osteoclastic differentiation and thereby modulate the biological actions of the molecular triad. In bone, RANKL is an important stimulator of bone resorption; it stimulates osteoclast formation, activation, adherence and survival upon binding to RANK which is expressed by the osteoclast and osteoclast precursors, as shown in figure 1. In the normal bone microenvironment, OPG binds to RANKL molecules as a decoy receptor, so that the interaction between RANKL and RANK gets inhibited, therefore OPG inhibits osteolysis, as shown in figure 1(4).
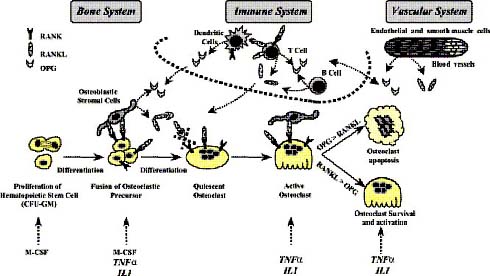
Figure 1: OPG/RANK/RANKL as common effectors of bone, immune and vascular systems (adapted from Theoleyre et al, 2004).
In multiple myeloma patients, myeloma cells cause bone marrow stromal cells to over-express RANKL for bone metastasis, whereas it decreases the OPG bio-availability in the bone microenvironment by expressing a gene called syndecan-1 on the surface of the myeloma cell. Syndecan-1 is a transmembrane proteoglycan which binds to the heparin-binding domain of OPG in the presence of heparan sulfates, and thereby internalizes and lysosomally degrades the OPG within the lysosomal compartment of myeloma cells. Hence myeloma cells disrupt the regulation of equilibrium between RANKL and OPG level in the bone microenvironment, resulting in improved RANKL to OPG ratio, thereby resulting in osteoclast formation and activation which is accountable for osteolysis, as shown in figure 2(5) (6).
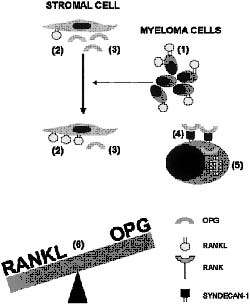
Figure 2: Interaction of the RANKL-OPG system with myeloma cells, bone marrow stromal cells and osteoclasts in the pathogenesis of myeloma bone disease (adapted from Sezer et al, 2003).
Current treatments for multiple myeloma
Chemotherapy
Drug chemotherapy is the systemic treatment for multiple myeloma (MM). Drug chemotherapy is the process of killing cancer cells using chemical drugs. The ultimate aim of chemotherapy is to control the cancer for as long as possible. One of the advantages of chemotherapy treatment is that it is very effective in killing tumour cells. However, it is not specific as it also kills healthy cells. It is a systemic treatment and is used in the treatment of lymphoma and leukaemia which are not restricted to one part of the body. The trouble associated with chemotherapy is that it distresses the normal body cells of hair follicles, bone marrow cells, the reproductive system and gastrointestinal tract, which causes poor blood clotting, vomiting, diarrhoea or constipation, nausea, and mouth and throat sores(7).
Thalidomide and Bortezomib – an everlasting drug in multiple myeloma treatment
Thalidomide is a successful drug with high response rates in the treatment of refractory and resistant multiple myeloma patients. The anti-tumour activity of thalidomide in multiple myeloma cells is a safe and effective method, but the mechanism of its action is not fully understood. Thalidomide exhibits its anti-tumour activity by the following mechanism: by blocking the blood flow to tumour cells (anti-angiogenesis) through inhibiting the factors like VEGF and basic fibroblast growth factors (bFGF), down-regulation of adhesion of MM cells to bone marrow stromal cells (BMSCs), G1 growth arrest of MM cells, shows a direct effect on MM cells/BM stromal cells via oxidative DNA damage, and blockage of cytokine secretion from MM cells and BMSCs, as shown in figure 3. This multi-faceted activity of thalidomide in the treatment of multiple myeloma has made it an attractive agent in single-agent chemotherapy or combinational chemotherapy treatment with corticosteroids(8).
An anabolic agent like bortezomib is the first representative class of drugs showing potent anti-myeloma activity by a significant elevation of alkaline phosphates (ALP) in the serum level. The rise in the serum ALP level is associated with stimulation of osteoblast differentiation and hence results in an anti-myeloma effect. Bortezomib is recommended for the treatment of refractory multiple myeloma patients. They are under investigation as a first-line therapy(9).
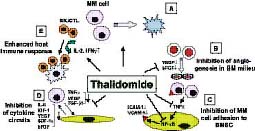
Figure 3: Pathways of thalidomide activity against multiple myeloma in the host microenvironment (adapted from Richardson et al, 2004).
Stem cell transplantation
Stem cell transplantation is a systemic treatment of multiple myeloma patients. It attains an elevated response rate and affords a considerable benefit to myeloma patients when comprehensive response is accomplished. Stem cell transplantation is done to support the high-dose chemotherapeutic treatment given to multiple myeloma patients. It provides a long duration of response with enhanced quality of life. Autologus peripheral blood stem cell transplantation treatment is frequently performed in multiple myeloma patients. Prior to high-dose chemotherapy, stem cells are isolated from the peripheral blood of an individual. The collected stem cells are frozen and stored if necessary. After high-dose chemotherapy, the same individual receives the stem cell transplant. In the case of allogeneic stem cell transplants, stem cells are isolated from an individual and given to other individuals who receive chemotherapeutic treatment. This type of allograft transplantation treatment is less frequent in the treatment of multiple myeloma patients(10) (12).
The benefits of peripheral blood stem cell transplants are that a reliable number of stem cells can be collected easily from peripheral blood cells. A quick recovery is achieved for multiple myeloma patients who are treated with peripheral blood stem cell transplants(10) (11).
Multiple myeloma treatment using autologous stem cell transplantation involves six major processes: initially, the stem cells are collected from the peripheral blood and then they are subjected to processing in the laboratory. The processed stem cells are frozen using liquid nitrogen at -196 degrees Celsius and stored until needed. The multiple myeloma patients undergo high-dose chemotherapy. After chemotherapeutic treatment, the stem cells are thawed and infused into the patient. The transplanted stem cells commence to synthesize new blood cells, as shown in figure 4(12).
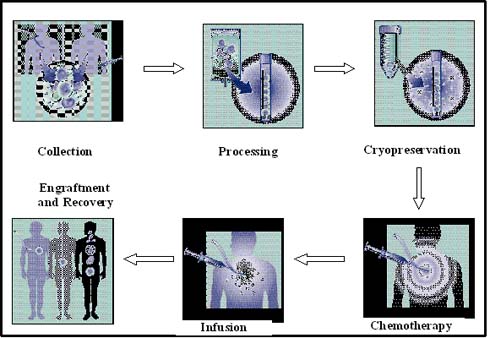
Figure 4: Stem cell transplantation process (adapted from Multiple Myeloma Research Foundation, 2004).
Bisphosphonate treatment
Bisphosphonates are drugs which inhibit osteoclast bone resorption activity and are effective in the treatment of bone metastasis. Bisphosphonates may directly exhibit the toxic effect on mature osteoclasts by inhibiting an enzyme in the mevalonate pathway, namely farnesyl diphosphate synthase (FPPS), which results in an alteration in osteoclast cytoskeletal integrity and thereby results in osteoclast apoptosis(13).
Recent studies by Vincent et al. (xxxx) emphasize that bisphosphonates like zoledronic acid or pamidronate can prevent osteolytic lesions, osteoporosis, and vertebral compression fractures(7). According to Berenson et al. (xxxx), intravenous delivery of pamidronate at a dose of 90 mg over 4 hours for every month reduces skeletal complication, reduces tumour growth and inhibits tumour-induced osteolysis formation in multiple myeloma patients(14). According to Rosen et al. (xxxx) and Berenson et al. (xxxx), zoledronic acid is more successful than pamidronate with a shorter infusion time of 15 minutes (15).
Immunotherapy
Immunotherapy is an active area of research in the treatment of multiple myeloma patients. Vaccines are manufactured to help the patient’s immune system to attack myeloma cells. Myelomas vaccines are synthesized from the patient’s own myeloma cells. The isolated myeloma cells are treated with the immune response stimulator (adjuvant) which elevates the body’s immune response against the multiple myeloma and makes a defence against it, as shown in figure 5. The various types of myeloma vaccines which are under current investigation are idiotype protein vaccines, DNA vaccines, gene-modified vaccines, cellular vaccines and dendritic cell vaccines. In addition to myeloma vaccine research, cytokines, monoclonal antibodies and alteration of immune cells are under investigation. The benefits of vaccines are that they are more specific to tumour cells and less toxic to patients than conventional therapies(16).
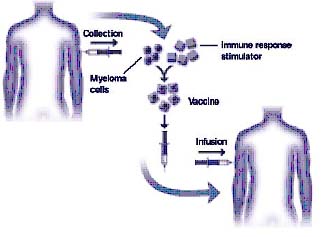
Figure 5: Development of myeloma vaccines (graphic courtesy of Freda K. Stevenson) (adapted from www.multiplemyeloma.org/treatments/3.08.13.html).
Novel treatments
Immunomodulatory drugs are identified as safer and more active drugs for the treatment of refractory myeloma. According to Richardson et al. (xxxx) & Zangari et al. (xxxx), an immuno-modulatory drug, namely CC-5013, has shown noteworthy activity in heavily pre-treated patients with refractory multiple myeloma disease in Phase I trials. Furthermore, proteasome inhibitor PS-341 used in the treatment of patients with refractory myeloma showed a response rate of 50%(17). According to Vincent et al. (xxxx), other active areas of investigation for the treatment of refractory myeloma include dendritic cell vaccination, inhibitors of angiogenic cytokines, 2-methoxyestradiol, flavopiridol and farnesyl transferase inhibitors(7).
Future treatments for multiple myeloma
Therapeutic targets of OPG, RANKL and RANK system
RANKL molecules are used as a potential therapeutic target in the treatment of myeloma bone disease. Osteolytic metastases are treated successfully by systemically blocking the RANKL molecules using recombinant RANKL antagonist sRANK – Fc and recombinant OPG fusion protein (OPG-Fc) molecules. sRANK – Fc is formed by the fusion of extra-cellular domain of soluble RANK to the Fc portion of IgG1 human immunoglobulin(18).
According to Pearse et al. (2001), an immuno-compromised mouse is injected with myeloma cells which are isolated from the bone marrow cells of myeloma patients. The injected mice are in turn intravenously injected with 200 micrograms of sRANK – Fc protein three times per week. As a result, bone resorption is prevented and there were distinctly fewer osteolytic lesions found when compared to the control, as shown in figure 6. This study revealed that sRANK – Fc protein may be a successful and efficient therapeutic option for diseases linked with extreme osteoclast activity. Intravenous injection of recombinant sRANK – Fc protein into the mice reduced the tumour burden and elevated the bone radio density(19).
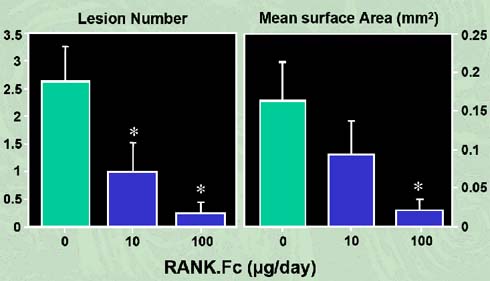
Figure 6: RANK-Fc inhibits the development of osteolytic lesions in myeloma-bearing mice (adapted from Oyajobi et al, 2005).
According to Sezer et al. (2003), the recombinant OPG-Fc protein binds with the RANKL molecules and thereby blocks the RANKL-RANK interaction in active osteoclasts hence reducing bone resorption. It is a more successful and effective treatment method in reducing bone resorption than bisphosphonates like pamidronate(6).
According to Croucher et al (xxxx), in a 5T2MM model, a syngeneic mouse is injected with murine 5T2MM myeloma cells. As a result, the syngeneic mice develop enhancive osteolytic lesions. In turn, the injected mice are intravenously injected with 30 mg/kg of OPG-Fc protein three times per week. As a result, there were distinctly fewer osteolytic lesions found when compared to the control. This study revealed that OPG-Fc protein not only prevented bone loss on subsequent injection of 5T2MM, but also increased bone mineral density and resulted in a complete non-appearance of osteoclasts, as shown in figure 7(20).
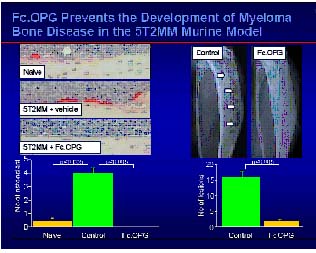
Figure 7: Fc-OPG prevents the development of myeloma bone disease in the 52TMM murine model (adapted from the slides of zolendric acid preclinical profile by Peter Croucher, University of Sheffield, UK, 2001).
According to Doran et al. (2004), myeloma cells are transfected (lenti-viral transfection) with native OPG-Fc gene and injected into immuno-compromised mice. This resulted in reduction in the development of lytic lesions, longer survival, reduced bone resorption and inhibited osteolytic lesions in the mice carrying OPG-Fc expressing tumours when compared to mice treated with the empty vector as control(21).
According to Onyia et al. (2004), an antiresorptive drug called AMG 162 (Amgen®; Amgen Inc., Thousand Oaks, CA) is a human monoclonal antibody against RANKL and thereby reduces bone resorption in post-menopausal women associated with multiple myeloma(22). High throughput screening methods are used to screen a small and selective compound called Cmpd 5 which is used to stimulate the OPG expression and reduce the bone resorption in mice treated with myeloma cell lines(22).
According to Pan et al. (2004), bisphosphonates, zoledronic acid can reduce the bio-availability of RANKL by decreasing the expression of RANKL on the surface of human osteoblast cells or by stimulating a TNF-α converting enzyme which cleaves the RANKL from the cell membrane. Also, bisphosphonates like zoledronic acid and pamidronate can stimulate the production of OPG by primary human osteoblasts in a dose-dependent manner. Combination therapy by anti-resorptive drug with anti-tumour agent leads to enhanced treatment of bone metastases(23).
Wnt signalling regulates the bone formation by osteoblastogenesis
The Wnt/β-catenin signalling pathway or the canonical pathway plays a vital role in the development and maintenance of bone. The canonical Wnt signalling is activated when the Wnt molecule binds and forms a complex within the receptor of low-density lipoprotein receptor-related protein (LRP5/6) and frizzleds receptor. Upon Wnt-receptor interaction, Axin and Frat1 are recruited to the membrane to form a juxtamembrane protein complex in addition with APC (adenomatous polyposis coli) and disheveled (Dsh). This large juxtamembrane protein complex inactivates the glycogen synthase kinase 3-beta (GSK-3β). The inactivated GSK-3β leads to stabilization and accumulation of its substrate β-catenin into cytoplasm. Upon translocation to the nucleus, β-catenin binds with the transcription factor called Lef1/Tcf, which alters the specific target gene expression, as shown in figure 8(24) (25).
Soluble Wnt antagonist can block the canonical pathway and encourage the degradation of β-catenin in two ways. Secreted frizzled-related proteins (Sfrps) can firmly bind with the free Wnt molecules and inhibit the Wnt molecules from binding with the receptors (i.e. interaction among Wnt with LRP5/6-frizzled surface receptor is inhibited). This results in activation of GSK3 molecules which phosphorylate the β-catenin and proteasomally degrade it. Alternatively, Dickkopfs (Dkk) molecule binds within the extra-cellular domain of the receptor Kremens (Kringle-coding genes marking the eye and the nose; Krms) and LRP5/6, followed by internalization of trimer complex into the lysosomes where LRP5/6 is degraded. Finally, the expression of LRP5/6 in the cell surface is decreased, hence Wnt signalling is disrupted, as shown in figure 8(24) (25).
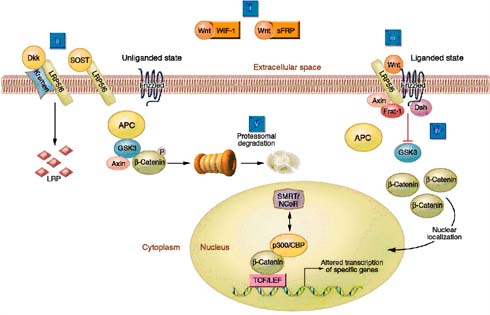
Figure 8: Elements of Wnt/beta-catenin signalling (adapted from Krishnan et al, 2006).
Wnt/β-catenin signalling enhances osteoblast differentiation, proliferation and mineralization. It strictly inhibits the process of osteoblast apoptosis, osteoclast differentiation and mesenchymal differentiation pathways into adipocyte differentiation and chondrocyte differentiation, as shown in figure 8. Wnt signalling regulates bone formation by osteoblastogenesis. Various form of Wnt like Wnt3a, Wnt1, Wnt10b and active β-catenin activate the process of osteoblastogenesis(24) (25). According to Jackson et al (2005), C3H10T1/2 mesenchymal cells treated with Wnt3a molecules induced the expression of BMP4, Ctgf and Lox genes, which are all important regulators of osteoblast function(26). According to Beninett et al. (2005), C3H10T1/2 mesenchymal cells were treated with Wnt10b molecules, which induced the expression of Runx2, Dlx5 and osterix, which are all important transcription factors of stimulating osteoblastogenesis, as shown in figure 9. Interestingly, Wnt signalling enhances the expression of OPG molecules and represses the expression of RANKL molecules, resulting in a negative impact on osteoclast function and differentiation(27).
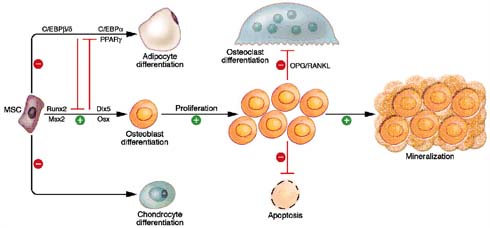
Figure 9: Wnt/ β-catenin signalling regulates osteogenesis through multiple mechanisms (adapted from Krishnan et al, 2006).
Conclusion
In skeletal biology, anabolic drugs play a vital role in targeting the Wnt signalling pathway for treating bone diseases. In adults, the bone formation rate can be altered by pharmacological activation of the Wnt cascade. In clinical practices, anabolic drugs therapy is introduced to reduce the risk of skeletal fractures in post-menopausal osteoporosis patients. A pharmaceutical product like terparatide, which is a human parathyroid hormone, is given to the osteoporosis patients to block bone resorption and stimulate a modest increase in bone mineral density by activating the Wnt/β-catenin pathway in bone. Identification and screening of drugs which specifically target the Wnt/β-catenin pathway in bone holds tremendous promise in the field of drug discovery(28). Secreted frizzled-related proteins (Sfrps) can firmly bind with the free Wnt molecules and inhibit the Wnt/β-catenin pathway in bone. Discovery of a small chemical compound or antibodies or peptides which tend to inhibit the Sfrps from binding with free Wnt molecules holds tremendous promise as anabolic agents in the treatment of bone disease(29).
References
- Bataille R, Harousseau JL. Multiple Myeloma. N Engl J Med. 1997; 336: 1657-1664
- Julie M Blair, Hong Zhou, Markus J Seibel and Colin R Dunstan (2006): Mechanisms of Disease: roles of OPG, RANKL and RANK in the pathophysiology of skeletal metastasis. Nature Clinical Practice Oncology .Vol.3 , No.1: 41-49
- Roodman GD (1996) Advances in bone biology: the osteoclast. Endocr Rev 17: 308-332
- Sandrine Theoleyre, Yohann Wittrant, Steeve Kwan Tat, Yannick Fortun, Francoise Redini, Dominique Heymann (2004): The molecular triad OPG/RANK/RANKL: involvement in the orchestration of pathophysiological bone remodelling. Cytokine & Growth Factor Reviews 15: 457-475
- Standal T, Seidel C, Hjertner O, et al (2002): Osteoprotegerin is bound, internalized and degraded by multiple myeloma cells. Blood; 100: 3002-3007
- Orhan Sezer, Ulrike Heider, Ivana Zavrski, Christian Alexander Kuhne, and Christian Hofbauer (2003): RANK ligand and osteoprotegerin in myeloma bone disease. Blood; Vol. 101, No.6:2094-2098
- S.Vincent Rajkumar, MD; Morie A. Gertz, MD; Robert A. Kyle, MD; AND Philip R. Greipp, MD (2002): Current therapy for multiple myeloma. Mayo Clin Proc. 77: 813-822
- Paul Richardson, Robert Schlossman, Sundar Jagannath, Melissa Alsina, Raman Desikan, Emily Blood, Edie Weller, Andrea Freeman, Joan Bosch, John Patin, Robert Knight, Jeromes Zeldis, and William Dalton, Kenneth Anderson (2004): Thalidomide for patients with relapsed multiple myeloma after high-dose chemotherapy and stem cell transplantation: Results of an open-label multicenter phase 2 study of efficacy, toxicity, and biological activity. Mayo Clin Proc. 79(7): 875-882
- Zangari M, Esseltine D, Lee CK, et al (2005). Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br J Haematol.; 131: 71-73.
- Attal M, Harousseau JL, Stoppa AM, et al. (1996). A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 335:91-97
- Fassas AB, Van Rhee F, Tricot G (2003). Predicting long-term survival in multiple myeloma patients following autotransplants. Leuk Lymphoma 44: 749-758
- Multiple Myeloma Research Foundation accelerates the search for a cure for multiple myeloma using stem cell transplantation (MMRF) 2004.
- Kyle RA (2000). The role of bisphosphonates in multiple myeloma. Ann Intern Med. 132:734-736
- Berenson JR, Lichtenstein A, Porter L, et al (1996), Myeloma Aredia Study Group. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. N Engl J Med. 334:488-493
- Rosen LS, Gordon D, Antonio BS, et al (2001). Zoledronic acid versus Pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: a phase III, double-blind, Comparative trial. Cancer. 91: 1191-1200
- Stevenson, F.K. and Anderson, K.C. (2002) Immunotherapy for multiple myeloma: insights from other models.Leukaemia Research 26, (4), 403-405.
- Richardson PG, Schlossman RL, Hideshima T, et al (2001). A phase I study of oral CC5013, an immunomodulatory thalidomide derivative, in patients with relapsed and refractory multiple myeloma. Blood;98: 775a
- Zlatibor Andelkovic, Vuka Katic, Dragan Mihailovic, Aleksandar Petrovic, Ivan Bubanovic (2004).RANKL/RANK/Osteoprotegerin system as novel therapeutic target in the treatment of primary bone tumours and osteolytic metastases. Arch Oncol; 12(2): 112-114
- Pearse RN, Sordillo EM, Yaccoby S, et al.(2001) Multiple myeloma disrupts the TRANCE/osteprotegerin cytokine axis to stimulate bone destruction and promote tumour progression. Proc Natl Acad Sci U.S.A. 98:11581-11586
- Croucher PI, Shipman CM, Lippitt J, et al (2001). Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 98:3534-3540
- Doran PM et al (2004). Native osteoprotegerin gene transfer inhibits the development of murine osteolytic bone disease induced by tumour xenografts. Exp Hematol 32: 351-359
- Oniya JE et al (2004). Novel and selective small molecule stimulators of osteoprotegerin expression inhibit bone resorption. J Pharmacol Exp Ther 309: 369-379
- Pan B et al. (2004). The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cell by activating TNF-alpha converting enzyme (TACE). J Bone Miner Res 19: 147-154
- Jennifer J. Westendorf, Rachel A. Kahler, Tania M.Schroeder (2004). Wnt signaling in osteoblasts and bone diseases.Gene and Genomes 341: 19-39
- Venkatesh Krishnan, Henry U. Bryant, and Ormond A. MacDougald (2006). Regulation of bone mass by Wnt signalling.Journal of Clinical Investigation; Vol 16: 1202-1209
- Jackson A, Vayssiere B, Garcia T et al.(2005) Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone14:14
- Bennett CN. Longo KA. Wright WS et al (2005): Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc. Natl. Acad.Sci. USA 102(9):3324-3329
- Nicoloa Giuliani, Vittorio Rizzoli, G.David Roodman (2006): Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood 12:261-268
- Georges Rawadi & Sergio Roman-Roman (2005): Wnt Signalling pathway: a new target for the treatment of osteoporosis. Expert Opinion Ther Targets 9(5): 1063-1077